cho 12(g) CH3COOH tác dụng với 6,9(g) C2H5OH thu được 8,8(g) CH3COOC2H5.xác định H=?
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


nNaOH=0,3(mol)
CH3COOH + NaOH -> CH3COONa + H2O
x__________x__________________x(mol)
CH3COOC2H5 + NaOH -> CH3COONa + C2H5OH
y_____________y(mol)
Hệ pt:
\(\left\{{}\begin{matrix}60x+88y=20,8\\x+y=0,3\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}x=0,2\\y=0,1\end{matrix}\right.\)
mH2O=18x=18.0,2=3,6(g) => V(H2O)=3,6(ml)
mC2H5OH=46y=46.0,1=4,6(g) => V(H2O)= 46/0,8=57,5(ml)
=> \(D_r=\dfrac{57,5}{57,5+3,6}.100\approx94,1^o\)

a) nCH3COOH= 0,4(mol)
PTHH: CH3COOH + NaOH -> CH3COONa + H2O
0,4____________0,4(mol)
=> mNaOH=0,4. 40=16(g)
b) nCH3COOH= 1(mol)
nC2H5OH= 100/46= 50/23(mol)
Vì : 1/1< 50/23 :1
=> C2H5OH dư, CH3COOH hết, tính theo nCH3COOH.
PTHH: CH3COOH + C2H5OH \(⇌\) CH3COOC2H5 + H2O (đk: H+ , nhiệt độ)
Ta có: nCH3COOC2H5(thực tế)= 0,625(mol)
Mà theo LT: nCH3COOC2H5(LT)= nCH3COOH=1(mol)
=>H= (0,625/1).100=62,5%

\(n_{CH_3COOH}=\dfrac{120}{60}=2\left(mol\right)\)
\(n_{C_2H_5OH}=\dfrac{46}{46}=1\left(mol\right)\)
\(CH_3COOH+C_2H_5OH⇌CH_3COOC_2H_5+H_2O\left(ĐK:H_2SO_{4\left(đ\right)},t^0\right)\)
\(Bđ:\) \(2.........................1\)
\(Pư:1.......................1.....................1\)
\(KT:1.....................0...................1\)
\(m_{CH_3COOC_2H_5}=1\cdot88=88\left(g\right)\)
\(H\%=\dfrac{52.8}{88}\cdot100\%=60\%\)

a, PTHH: CH3COOH + C2H5OH --to, H2SO4(đ)--> CH3COOC2H5 + H2O
b, \(\left\{{}\begin{matrix}n_{CH_3COOH}=\dfrac{30}{60}=0,5\left(mol\right)\\n_{C_2H_5OH}=\dfrac{80}{46}=\dfrac{40}{23}\left(mol\right)\end{matrix}\right.\)
LTL: \(0,5< \dfrac{40}{23}\) => C2H5OH dư
Theo pthh: nCH3COOC2H5 = nCH3COOH = 0,5 (mol)
=> \(m_{este}=0,5.80\%.88=35,2\left(g\right)\)
\(CH_3COOH+C_2H_5OH\underrightarrow{H_2SO_4đ,t^o}CH_3COOC_2H_5+H_2O\)
\(nCH_3COOH=\dfrac{30}{60}=0,5\left(mol\right)\)
\(nC_2H_5OH=\dfrac{80}{46}=1,74\left(mol\right)\)
=> CH3COOH đủ , nC2H5OH dư
=> \(CH_3COOC_2H_5=0,5\left(mol\right)\)
=> \(mCH_3COOC_2H_5=0,5.88=44\left(g\right)\)
=> \(mCH_3COOC_2H_{5\left(thựcte\right)}=\dfrac{44.80}{100}=35,2\left(g\right)\)
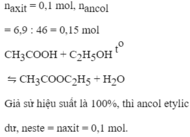
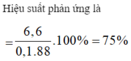


CH3COOh+C2H5OH->CH3COOC2H5+H2O
n CH3COOH=0,2 mol
n C2H5OH=0,15 mol
=>CH3COOh du
n CH3COOC2H5 tt=0,1 mol
=>H=\(\dfrac{0,1}{0,15}100=66,67\%\)