Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(2KClO_3\xrightarrow[]{t^o}2KCl+3O_2\)
\(n_{O_2}=\dfrac{V_{O_2}}{22,4}=\dfrac{5,6}{22,4}=0,25\left(mol\right)\)
Theo PTHH: \(n_{KClO_3}=\dfrac{0,25.2}{3}\approx0,17\left(mol\right)\)
Vậy muốn điều chế 5,6 lít O2 cần dùng số gam Kali clorat:
\(m_{KClO_3}=n_{KClO_3}.M_{KClO_3}=0,17.122,5=20,825g\)
\(n_{O2}\)=\(\dfrac{V}{22,4}\)=\(\dfrac{5,6}{22,4}\)=0,25 (mol)
PT : 2KClO3 →to 2KCl + 3O2
số mol: \(\dfrac{1}{6}\) ← \(\dfrac{1}{6}\) ← 0,25
⇒ mKClO3 = n . M = \(\dfrac{1}{6}\) . 122,5 ∼∼ 20,41(g)

\(n_{O_2}=\dfrac{5,6}{22,4}=0,25\left(mol\right)\)
PT: \(2KClO_3\underrightarrow{t^o}2KCl+3O_2\)
Theo PT: \(n_{KClO_3}=\dfrac{2}{3}n_{O_2}=\dfrac{1}{6}\left(mol\right)\Rightarrow m_{KClO_3}=\dfrac{1}{6}.122,5=\dfrac{245}{12}\left(g\right)\)

a) \(n_{O_2}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\)
PTHH: 2KClO3 --to--> 2KCl + 3O2
0,2<-------------------0,3
=> \(m_{KClO_3}=0,2.122,5=24,5\left(g\right)\)
b) \(n_{KClO_3}=\dfrac{490}{122,5}=4\left(mol\right)\)
PTHH: 2KClO3 --to--> 2KCl + 3O2
4-------------->4---->6
=> \(m_{KCl}=4.74,5=298\left(g\right)\)
=> \(m_{O_2}=6.32=192\left(g\right)\)
2KClO3 \(\underrightarrow{t^o}\) 2KCl + 3O2
a, \(n_{O_2}=\dfrac{6,72}{22,4}=0,3mol\\ n_{KClO_3}=\dfrac{0,3.2}{3}=0,2mol\\ m_{KClO_3}=0,2.122,5=24,5g\)
b, \(n_{KClO_3}=\dfrac{490}{122,5}=4mol\)
\(\Rightarrow m_{KCl}=4.74,5=298g\)
\(n_{O_2}=\dfrac{4.3}{2}=6mol\\ m_{O_2}=6.32=192g\)

\(a.n_{O_2}=\dfrac{4,48}{22,4}=0,2\left(mol\right)\\ \Rightarrow n_{KClO_3}=0,2.\dfrac{2}{3}=\dfrac{2}{15}\left(mol\right)\\ m_{KClO_3}=\dfrac{2}{15}.122,5\approx16,333\left(g\right)\\ b.n_{KClO_3}=1,5\left(mol\right)\Rightarrow n_{O_2}=\dfrac{3}{2}.1,5=2,25\left(mol\right)\\ m_{O_2}=2,25.32=144\left(g\right)\\ c.n_{KClO_3}=0,1\left(mol\right)\\ \Rightarrow n_{KCl}=n_{KClO_3}=0,1\left(mol\right);n_{O_2}=\dfrac{3}{2}.0,1=0,15\left(mol\right)\)

1)
H2+CuO->Cu+H2O
0,2-----------0,2 mol
nH2=\(\dfrac{4,48}{22,4}\)=0,2 mol
=>m Cu=0,2.64=12,8g
2)
2KClO3-to>2KCl+3O2
0,3----------------------0,45 mol
n KClO3=\(\dfrac{36,75}{122,5}\)=0,3 mol
=>VO2=0,45.22,4=10,08l
3Fe+2O2-to>Fe3O4
0,675--0,45 mol
=>m Fe=0,675.56=37,8g

a) 2KClO3 (7/75 mol) \(\underrightarrow{t^o}\) 2KCl (7/75 mol) + 3O2\(\uparrow\) (0,14 mol).
b) Số mol khí oxi là 4,48/32=0,14 (mol).
Khối lượng kali clorat cần dùng là 7/75.122,5=343/30 (g).
Khối lượng chất rắn thu được là 7/75.74,5=1043/150 (g).
\(a,PTHH:2KClO_3\underrightarrow{t^o,MnO_2}2KCl+3O_2\uparrow\\ b,n_{O_2}=\dfrac{4,48}{22,4}=0,2\left(mol\right)\\ Theo.pt:n_{KClO_3}=\dfrac{2}{3}n_{O_2}=\dfrac{2}{3}.0,2=\dfrac{2}{15}\left(mol\right)\\ m_{KClO_3}=\dfrac{2}{15}.122,5=\dfrac{49}{3}\left(g\right)\)

\(n_{Fe_3O_4}=\dfrac{m}{M}=\dfrac{4,64}{232}=0,02mol\)
\(3Fe+2O_2\rightarrow\left(t^o\right)Fe_3O_4\)
0,06 0,04 0,02 ( mol )
\(m_{Fe}=n_{Fe}.M_{Fe}=0,06.56=3,36g\)
\(V_{O_2}=n_{O_2}.22,4=0,04.22,4=0,896l\)
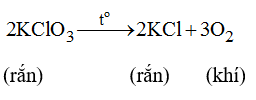
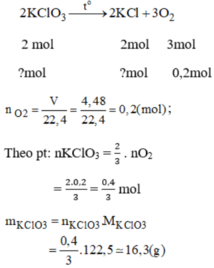

a)2KClO3------>2KCl+3O2
b) n O2=5,6/22,4=0,25(mol)
Theo pthh
n KClO3=2/3n O2=0,1667(mol)
m KClO3=0,1667.122,5=20,42(g)
n KCl=2nO2=0,1667(mol)
m KCl=0,166.74,5=12,42(g)
\(a,PTHH:2KClO_3\rightarrow2KCl+3O_2\)
\(b,n_{O_2}=\frac{m_{O_2}}{22,4}=\frac{5,6}{22,4}=0,25\left(mol\right)\)
\(\Rightarrow n_{KClO_3}=0,25.\frac{2}{3}=\frac{0,5}{3}\left(mol\right)\)
\(n_{KClO_3}=\frac{0,5}{3}.112,5=20,42\left(g\right)\)
Vậy ........