Cho 18,7 gam hỗn hợp A gồm Na và K2O tan hết trong 181,5 gam nước thu được 200 gam dung dịch X. Tính nồng độ phần trăm của mỗi chất tan trong dung dịch X?
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


a)
\(n_{H_2}=\dfrac{0,336}{22,4}=0,015\left(mol\right)\)
PTHH: 2Na + 2H2O --> 2NaOH + H2
0,03<------------0,03<----0,015
=> \(\%m_{Na}=\dfrac{0,03.23}{1,31}.100\%=52,67\%\)
=> \(\%m_{Na_2O}=100\%-52,67\%=47,33\%\)
b)
\(n_{Na_2O}=\dfrac{1,31.47,33\%}{62}=0,01\left(mol\right)\)
PTHH: Na2O + H2O --> 2NaOH
0,01----------->0,02
=> nNaOH = 0,03 + 0,02 = 0,05 (mol)
mdd sau pư = 1,31 + 18,72 - 0,015.2 = 20 (g)
=> \(C\%_{dd.NaOH}=\dfrac{0,05.40}{20}.100\%=10\%\)
\(V_{dd.NaOH}=\dfrac{20}{1,2}=\dfrac{50}{3}\left(ml\right)=\dfrac{1}{60}\left(l\right)\)
\(C_{M\left(dd.NaOH\right)}=\dfrac{0,05}{\dfrac{1}{60}}=3M\)

a)
$2K + 2H_2O \to 2KOH + H_2$
$BaO + H_2O \to Ba(OH)_2$
Theo PTHH :
$n_K = 2n_{H_2} = 0,2(mol)$
$\%m_K = \dfrac{0,2.39}{23,1}.100\% = 33,77\%$
$\%m_{BaO} = 100\%- 33,77\% = 66,23\%$
b)
$n_{BaO} = \dfrac{23,1 - 0,2.39}{153} = 0,1(mol)$
$m_{dd} = 23,1 + 177,1 - 0,1.2 = 200(gam)$
$C\%_{KOH} = \dfrac{0,2.56}{200}.100\% = 5,6\%$
$C\%_{Ba(OH)_2} = \dfrac{0,1.171}{200}.100\% = 8,55\%$
c)
$KOH + HCl \to KCl + H_2O$
$Ba(OH)_2 + 2HCl \to BaCl_2 + 2H_2O$
$n_{HCl} = 2n_{Ba(OH)_2} + n_{KOH} = 0,4(mol)$
$V = \dfrac{0,4}{0,5} = 0,8(lít) = 800(ml)$

2Fe(OH)3 -----to---> Fe2O3 + 3H2O
Mg(OH)2 ----to---> MgO + H2O
Gọi x, y lần lượt là số mol Fe(OH)3 và Mg(OH)2
\(\left\{{}\begin{matrix}107x+58y=16,5\\\dfrac{1}{2}.160x+y.40=12\end{matrix}\right.\)
=> x=0,1 ; y=0,1
\(\%m_{Fe\left(OH\right)_3}=\dfrac{107.0,1}{16,5}.100=64,85\%\)
%Mg(OH)2 = 35,15%
b) \(2Fe\left(OH\right)_3+3H_2SO_4\rightarrow Fe_2\left(SO_4\right)_3+6H_2O\)
0,1----------------------------------->0,05
\(Mg\left(OH\right)_2+H_2SO_4\rightarrow MgSO_4+2H_2O\)
0,1------------------------------------>0,1
\(m_{ddsaupu}=16,5+200=216,5\left(g\right)\)
\(C\%_{Fe_2\left(SO_4\right)_3}=\dfrac{0,05.400}{216,5}.100=9,24\%\)
\(C\%_{MgSO_4}=\dfrac{0,1.12}{216,5}.100=5,54\%\)

\(m_{H_2} = 8,5 + 50 - 58,4 = 0,1(gam)\\ n_{H_2} = \dfrac{0,1}{2} = 0,05(mol)\\ 2Na + 2H_2O \to 2NaOH + H_2\\ Na_2O + H_2O \to 2NaOH\\ n_{Na} = 2n_{H_2} = 0,05.2 = 0,1(mol)\\ \Rightarrow n_{Na_2O} = \dfrac{8,5-0,1.23}{62}=0,1(mol)\\ n_{NaOH} = 2n_{Na_2O} + n_{Na} = 0,3(mol)\\ C\%_{NaOH} = \dfrac{0,3.40}{58,4}.100\% = 20,55\%\)

2Na + 2H2O \(\rightarrow\) 2NaOH + H2 (1)
K2O + H2O \(\rightarrow\) 2KOH (2)
Có : mdd B = mhh A + mH2O - mH2 = 19,85 + 180,4 - mH2 = 200
\(\Rightarrow\) mH2 = 0,25(g)
\(\Rightarrow\) nH2 = 0,25/2 = 0,125(mol)
Theo PT(1) \(\Rightarrow\)nNa = nNaOH = 2.nH2 = 2. 0,125 = 0,25(mol)
\(\Rightarrow\left\{{}\begin{matrix}m_{Na}=0,25.23=5,75\left(g\right)\\m_{NaOH}=0,25.40=10\left(g\right)\end{matrix}\right.\)
\(\Rightarrow\) mK2O = 19,85 - 5,75= 14,1(g)
\(\Rightarrow\) nK2O = 14,1/94 = 0,15(mol)
Theo PT(2) \(\Rightarrow\) nKOH = 2 . nK2O = 2. 0,15 = 0,3(mol)
\(\Rightarrow\) mKOH = 0,3 . 56 = 16,8(g)
* C%KOH / ddB = 16,8/200 . 100% = 8,4%
C%NaOH / dd B = 10/200 . 100% = 5%
* m(KOH+ NaOH) = 16 ,8 + 10 =\ 26,8(g)
\(\Rightarrow\)mH2O / dd B = 200 - 26,8 = 173,2 (g)
\(\Rightarrow\) VH2O / dd B = m : D = 173,2 : 1 = 173,2 (ml) =0,1732(l)
mà Vdd B = VH2O / ddB
=> Vdd B =\ 0,1732(l)
Do đó :
CM của NaOH / dd B = 0,25/0,1732=1,44(M)
CM của KOH / dd B = 0,3/0,1732 = 1,73 (M)

a,Fe + 2HCl → FeCl + H2 (1)
FeO + 2HCl → FeCl + H2O (2)
nH2 = 3,36/ 22,4 = 0,15 ( mol)
Theo (1) nH2 = nFe = 0,15 ( mol)
mFe = 0,15 x 56 = 8.4 (g)
m FeO = 12 - 8,4 = 3,6 (g)
a, \(n_{H_2}=\frac{3,36}{22,4}=0,15\left(mol\right)\)
\(Fe+2HCl->FeCl_2+H_2\left(1\right)\)
\(FeO+2HCl->FeCl_2+H_2O\left(2\right)\)
theo (1) \(n_{Fe}=n_{H_2}=0,15\left(mol\right)\)
=> \(m_{Fe}=0,15.56=8,4\left(g\right)\)
=> \(m_{FeO}=12-8,4=3,6\left(g\right)\)

ta thấy : nFe =nH2 = 0,15
=> mFe =0,15 x 56 = 8,4g
%Fe=8,4/12 x 100 = 70%
=>%FeO = 100 - 70 = 30%
b) BTKLra mdd tìm mct of HCl
c) tìm mdd sau pứ -mH2 nha bạn
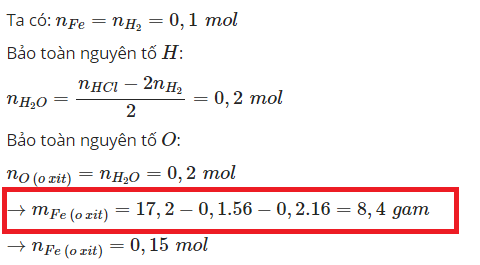
\(n_{Na}=x\left(mol\right)\)
\(n_{K_2O}=y\left(mol\right)\)
\(m_{hhA}=23x+94y=18,7\left(I\right)\)
PTHH:
2Na + 2H2O \(\rightarrow\) 2NaOH + H2\(\uparrow\) (1)
(mol) x...........................x..............0,5x
K2O + H2O \(\rightarrow\) 2KOH (2)
(mol) y..........................y
\(m_{hhX}=m_{H_2O}+m_{hhA}-m_{H_2\uparrow}\)
\(200=181,5+18,7-m_{H_2\uparrow}\)
\(m_{H_2\uparrow}=0,2\left(g\right)\)
\(n_{H_2}=\dfrac{0,2}{2}=0,1\left(mol\right)\)
\(\left(1\right)\rightarrow n_{H_2}=0,5.x=0,1\)
\(\rightarrow x=0,2\left(mol\right)\)
\(\left(I\right)\rightarrow y=0,15\left(mol\right)\)
200(g) ddX có 2 chất tan: NaOH, KOH
\(\left(1\right)\rightarrow n_{NaOH}=x=0,2\left(mol\right)\)
\(\left(2\right)\rightarrow n_{KOH}=2y=0,3\left(mol\right)\)
\(C\%_{NaOH/_{ddX}}=\dfrac{40.0,2}{200}.100=4\%\)
\(C\%_{KOH/_{ddX}}=\dfrac{56.0,3}{200}.100=8,4\%.\)