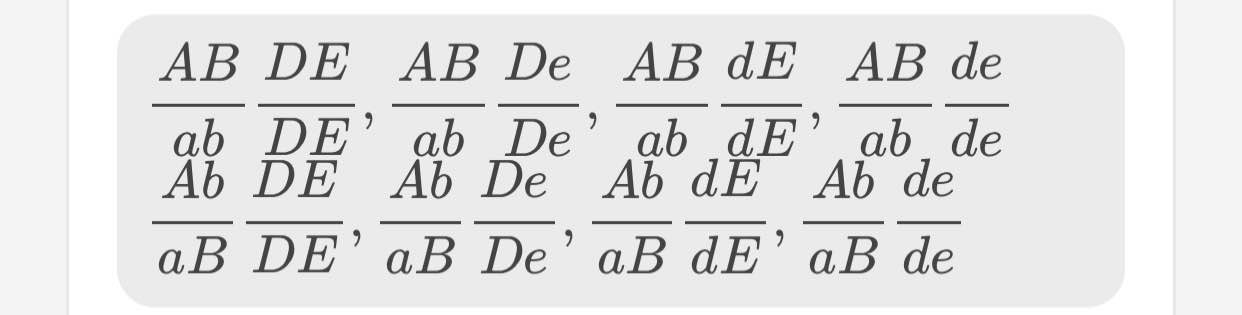Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.



Bài 5:
1. There are a few students taking part in the event
→ There aren't many students taking part in the event.
2. If he doesn't work hard, he will lose his job
→ Unless he works hard, he will lose his job.
3. Let's write about the three Rs ?
→ Why don't we write about the three Rs?
4. Learning about recyking is fun
→ It's fun to learn about recycling.
5. It's not good to throw old clothes away
→ You shouldn't throw old clothes away
Xếp từ thành câu
Bài 1
1. they / to / movies / do / how / go / the / often ?
→ How often do they go to the movies?
2. your eyes / swimming / should / when goggles / you / you / go / wear / to protect
→ When you go swimming, you should wear goggles to protect your eyes.
3. usually / his / swimming / with / friends / he / goes
→ He usually goes swimming with his friends.
4. go / do / weekend / on / always / fishing / parents / their ?
→ Do their parents always go fishing on the weekend?
5. camping / they / go / do / sometimes
→ Sometimes they go camping.
6. what / TV / you / do / on / sports / watch?
→ What sports do you watch on TV?
Bài 2
1. Our / important / an / sports and games / in play / lives / part
→ Sports and games play an important part in our lives.
2. players / how / match / there / in / many / are / football / a ?
→ How many players are there in a football match?
3. by / she / to keep / every day / tries / fit / jogging
→ She tries to keep fit by jogging every day.
4. yesterday / who / play / football / you / did / with ?
→ Who did you play football with yesterday?
5. sports / building / physical and strength / necessary / are / for
→ Sports are necessary for building physical strength.
6. to switch / before / go / don't / the TV / off / you / forget / to bed
→ Don't forget to switch off the TV before you go to bed.
7. Sunday / I / usually / friends / swimming / on / go / mornings / with / my
→ On Sunday mornings, I usually go swimming with my friends.
8. match / you / on / the / did / television / last night / watch / basketball / the ?
→ Did you watch the basketball match on television last night?

Bài 1:
c) \(C=\dfrac{5}{\sqrt{7}+\sqrt{2}} - \sqrt{8-2\sqrt{7}} + \sqrt{2} \)
⇔ \(C=\dfrac{5}{\sqrt{7}+\sqrt{2}} - \sqrt{(\sqrt{7})^2 - 2\sqrt{7}+1} + \sqrt{2} \)
⇔ \(C=\dfrac{5}{\sqrt{7}+\sqrt{2}} - \sqrt{(\sqrt{7}-1)^2} + \sqrt{2} \)do
⇔ \(C=\dfrac{5}{\sqrt{7}+\sqrt{2}} - |\sqrt{7}-1| + \sqrt{2} \)
⇔ \(C=\dfrac{5}{\sqrt{7}+\sqrt{2}} - \sqrt{7}+1 + \sqrt{2} \) (do \(\sqrt{7} > 1 \))
⇔ \(C=\dfrac{5}{\sqrt{7}+\sqrt{2}} - (\sqrt{7} - \sqrt{2}) +1 \)
⇔ \(C=\dfrac{5-(\sqrt{7} - \sqrt{2})(\sqrt{7}+\sqrt{2})}{\sqrt{7}+\sqrt{2}} +1 \)
⇔ \(C=\dfrac{5-7+2}{\sqrt{7}+\sqrt{2}} +1 =\dfrac{0}{\sqrt{7}+\sqrt{2}} +1 \)
⇔ \(C = 0 + 1 = 1\)
Vậy \(C=1\)
Bài 3:
c) Ta có: \(M=\dfrac{Q}{P} \)
⇔ \(M=\dfrac{\dfrac{\sqrt{x}}{\sqrt{x}-2}}{\dfrac{\sqrt{x}+5}{\sqrt{x}-2} } \)
⇔ \(M=\dfrac{\sqrt{x}}{\sqrt{x}+5} \)
Mà: \(M<\dfrac{1}{2} \) ⇔ \(\dfrac{\sqrt{x}}{\sqrt{x}+5} <\dfrac{1}{2} \)
⇒ \(2\sqrt{x} < \sqrt{x}+5 \) (nhân 2 vế với \(2.(\sqrt{x} +5) >0\))
⇔ \(\sqrt{x}<5 \) ⇔ \(x<25\)
Kết hợp điều kiện ban đầu, ta đc:
Vậy khi \(0≤x<25\) và \(x≠4\) thì \(M=\dfrac{Q}{P} < \dfrac{1}{2} \)

didn't stay
Cấu trúc thì quá khứ đơn
(-) S + did not + V(không chia).

\(\dfrac{AB}{ab}\dfrac{DE}{DE},\dfrac{AB}{ab}\dfrac{De}{De},\dfrac{AB}{ab}\dfrac{dE}{dE},\dfrac{AB}{ab}\dfrac{de}{de}\)
\(\dfrac{Ab}{aB}\dfrac{DE}{DE},\dfrac{Ab}{aB}\dfrac{De}{De},\dfrac{Ab}{aB}\dfrac{dE}{dE},\dfrac{Ab}{aB}\dfrac{de}{de}\)

a.b.\(n_P=\dfrac{m_P}{M_P}=\dfrac{6,2}{31}=0,2mol\)
\(n_{O_2}=\dfrac{V_{O_2}}{22,4}=\dfrac{6,72}{22,4}=0,3mol\)
\(4P+5O_2\rightarrow\left(t^o\right)2P_2O_5\)
0,2 < 0,3 ( mol )
0,2 0,25 0,1 ( mol )
Chất còn dư là O2
\(V_{O_2\left(dư\right)}=n_{O_2\left(dư\right)}.22,4=\left(0,3-0,25\right).22,4=1,12l\)
\(m_{P_2O_5}=n_{P_2O_5}.M_{P_2O_5}=0,1.142=14,2g\)
c.\(2KClO_3\rightarrow\left(t^o,MnO_2\right)2KCl+3O_2\)
1/6 0,25 ( mol )
\(m_{KClO_3}=n_{KClO_3}.M_{KClO_3}=\dfrac{1}{6}.122,5=20,41g\)
a) PTHH: \(4P+5O_2\rightarrow^{t^0}2P_2O_5\)
b) \(n_P=\dfrac{m}{M}=\dfrac{6,2}{31}=0,2\left(mol\right);n_{O_2}=\dfrac{V}{22,4}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\)
\(4P+5O_2\rightarrow^{t^0}2P_2O_5\)
4 : 5 : 2
0,2 : 0,3
-So sánh tỉ lệ: \(\dfrac{0,2}{4}< \dfrac{0,3}{5}\)
\(\Rightarrow\)P phản ứng hết còn O2 dư.
\(m_{O_2\left(dư\right)}=16.0,3-16.\dfrac{0,2.5}{4}=0,8\left(g\right)\)
c) -Theo PTHH trên:
\(n_{P_2O_5}=\dfrac{0,2.2}{4}=0,1\left(mol\right)\)
\(\Rightarrow m_{P_2O_5}=n.M=142.0,1=14,2\left(g\right)\)
d) -Theo PTHH trên:
\(n_{O_2\left(LT\right)}=\dfrac{0,2.5}{4}=0,25\left(mol\right)\)
PTHH: \(2KClO_3\rightarrow^{t^0}2KCl+3O_2\uparrow\)
2 : 2 : 3
\(\dfrac{1}{6}\) : \(\dfrac{1}{6}\) : 0,25
\(\Rightarrow m_{KClO_3}=n.M=\dfrac{1}{6}.122,5=\dfrac{245}{12}\left(g\right)\)




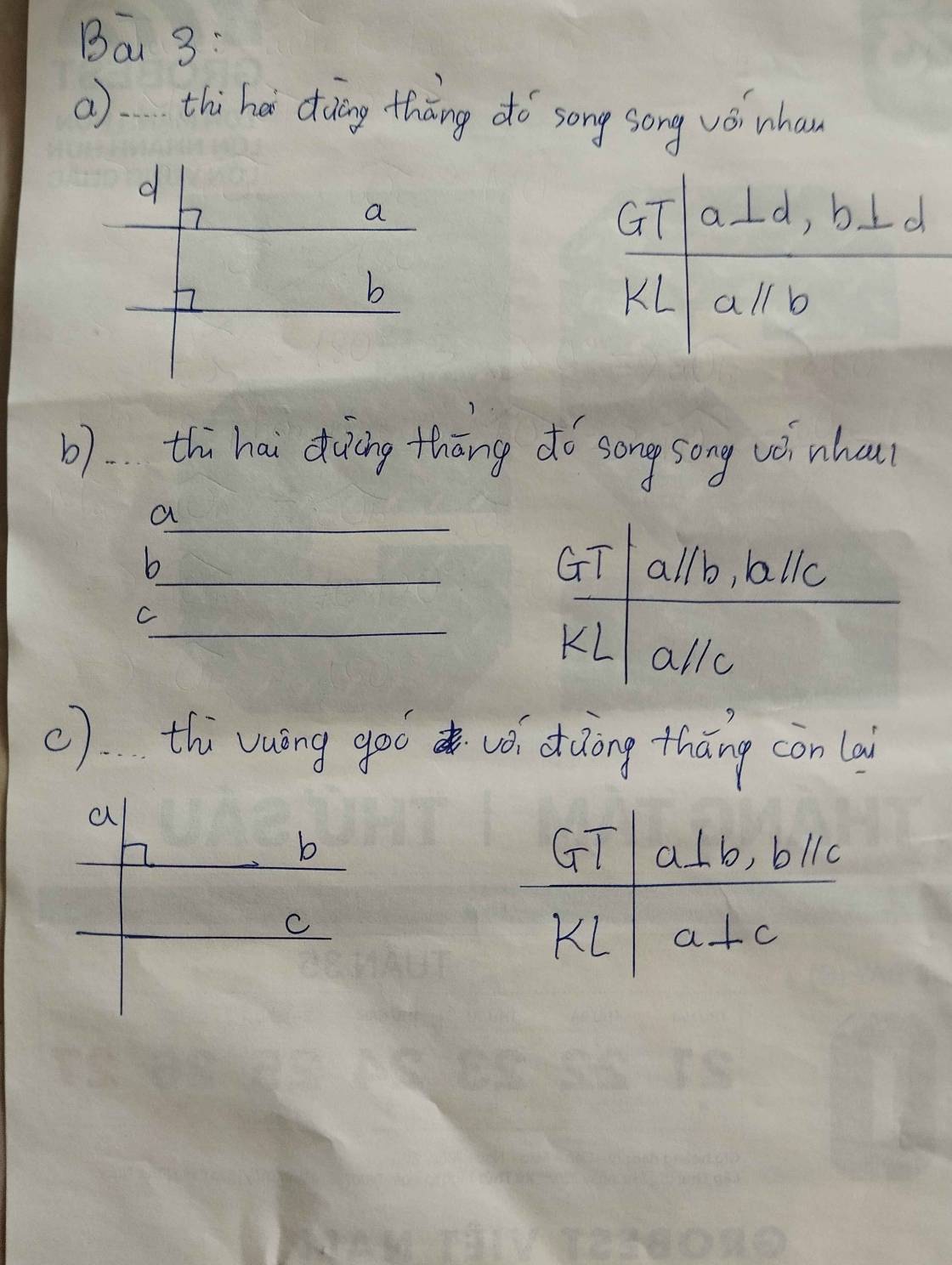
 giúp em 3 bài này với ạ em đang cần gấp chiều em hc ròi ạ ai làm đc bài nào thì gửi luôn giúp em ạ
giúp em 3 bài này với ạ em đang cần gấp chiều em hc ròi ạ ai làm đc bài nào thì gửi luôn giúp em ạ