Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

PTHH: \(Fe_2O_3+3H_2\underrightarrow{t^o}2Fe+3H_2O\)
a+b) \(n_{Fe}=\dfrac{22,4}{56}=0,4\left(mol\right)\)
\(\Rightarrow\left\{{}\begin{matrix}n_{Fe_2O_3}=0,2\left(mol\right)\\n_{H_2}=0,6\left(mol\right)\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}m_{Fe_2O_3}=0,2\cdot160=32\left(g\right)\\V_{H_2}=0,6\cdot22,4=13,44\left(l\right)\end{matrix}\right.\)
c) PTHH: \(Zn+H_2SO_4\rightarrow ZnSO_4+H_2\uparrow\)
Theo PTHH: \(n_{Zn}=n_{H_2}=0,6\left(mol\right)\)
\(\Rightarrow m_{Zn}=0,6\cdot65=39\left(g\right)\)
a,
nFe = 22,4/56 = 0,4 (mol)
PTHH
Fe2O3 + 3H2 ---to----) 2Fe + 3H2O (1)
theo phương trình (1) ,ta có:
nFe2O3 = 0,4 x 2 / 1 = 0,8 (mol)
mFe2O3 = 160 x 0,8 = 128 (g)
b,
theo pt (1)
nH2 = (0,4 x 3)/2 = 0,6 (mol)
=) VH2 = 0,6 x 22,4 = 13,44 (L)
c,
PTHH
Zn + H2SO4 -------------) ZnSO4 + H2 (2)
Số mol H2 cần dùng là 0,6 (mol)
Theo PT (2) :
nZn = nH2 ==) nZn = 0,6 x 65 = 39 (g)

a) \(H_2SO_4+Fe\rightarrow FeSO_4+H_2\)
\(n_{H_2SO_4}=\dfrac{m_{H_2SO_4}}{M_{H_2SO_4}}=\dfrac{49}{98}=0,5\left(mol\right)\)
Theo PTHH: \(n_{Fe}=n_{H_2SO_4}=0,5\left(mol\right)\)
\(\Rightarrow m_{Fe}=n_{Fe}.M_{Fe}=0,5.56=28\left(g\right)\)
b) Theo PTHH: \(n_{H_2}=n_{H_2SO_4}=0,5\left(mol\right)\)
\(\Rightarrow V_{H_2}=n_{H_2}.22,4=0,5.22,4=11,2\left(l\right)\)

a) $2KMnO_4 \xrightarrow{t^o} K_2MnO_4 + MnO_2 + O_2$
b) n KMnO4 = 15,8/158 = 0,1(mol)
Theo PTHH : n O2 = 1/2 n KMnO4 = 0,05(mol)
=> V O2 = 0,05.22,4 = 1,12(lít)
c)
$3Fe + 2O_2 \xrightarrow{t^o} Fe_3O_4$
Theo PTHH : n Fe = 3/2 nO2 = 0,075(mol)
=> m Fe = 0,075.56 = 4,2(gam)

a.\(n_{HCl}=\dfrac{10,95}{36,5}=0,3mol\)
\(2Al+6HCl\rightarrow2AlCl_3+3H_2\)
0,3 0,15 ( mol )
\(V_{H_2}=0,15.22,4=3,36l\)
b.\(n_{Fe_2O_3}=\dfrac{12}{160}=0,075mol\)
\(Fe_2O_3+3H_2\rightarrow\left(t^o\right)2Fe+3H_2O\)
\(\dfrac{0,075}{1}\) > \(\dfrac{0,15}{3}\) ( mol )
0,15 0,1 ( mol )
\(m_{Fe}=0,1.56=5,6g\)
\(2Al+6HCl\rightarrow2AlCl_3+3H_2\uparrow\)
1 : 6 : 2 : 3 (mol)
0,05 : 0,3 : 0,1 : 0,15 (mol)
\(n_{HCl}=\dfrac{m}{M}=\dfrac{10,95}{36,5}=0,3\left(mol\right)\)
a. \(V_{H_2\left(đktc\right)}=n.24,79=0,15.24,79=3,7185\left(l\right)\)
b. \(Fe_2O_3+3H_2\rightarrow^{t^0}2Fe+3H_2O\)
1 : 3 : 2 : 3 (mol)
0,075 : 0,15 (mol)
\(n_{Fe_2O_3}=\dfrac{m}{M}=\dfrac{12}{160}=0,075\left(mol\right)\)
-Chuyển thành tỉ lệ: \(\dfrac{0,075}{1}>\dfrac{0,15}{3}=0,05\)
\(\Rightarrow\)H2 phản ứng hết còn Fe2O3 dư.
\(Fe_2O_3+3H_2\rightarrow^{t^0}2Fe+3H_2O\)
1 : 3 : 2 : 3 (mol)
0,05 : 0,15 : 0,1 : 0,15 (mol)
\(\Rightarrow m_{Fe}=n.M=0,1.56=5,6\left(g\right)\)

Sửa đề: 4,46 (g) → 4,64 (g)
a, \(3Fe+2O_2\underrightarrow{t^o}Fe_3O_4\)
\(n_{Fe_3O_4}=\dfrac{4,64}{232}=0,02\left(mol\right)\)
Theo PT: \(n_{Fe}=3n_{Fe_3O_4}=0,06\left(mol\right)\Rightarrow m_{Fe}=0,06.56=3,36\left(g\right)\)
\(n_{O_2}=2n_{Fe_3O_4}=0,04\left(mol\right)\Rightarrow m_{O_2}=0,04.32=1,28\left(g\right)\)
b, \(2KMnO_4\underrightarrow{t^o}K_2MnO_4+MnO_2+O_2\)
\(n_{KMnO_4}=2n_{O_2}=0,08\left(mol\right)\Rightarrow m_{KMnO_4}=0,08.158=12,64\left(g\right)\)

$1)$
$PTHH:Ca(OH)_2+2HCl\to CaCl_2+2H_2O$
$n_{Ca(OH)_2}=\dfrac{14,8}{74}=0,2(mol)$
$n_{HCl}={10,95}{36,5}=0,3(mol)$
Lập tỉ lệ: $\dfrac{n_{Ca(OH)_2}}{1}>\dfrac{n_{HCl}}{2}\Rightarrow Ca(OH)_2$ dư
$\Rightarrow n_{Ca(OH)_2(dư)}=0,2-\dfrac{1}{2}.0,3=0,05(mol)$
Theo PT: $n_{CaCl_2}=\dfrac{1}{2}n_{HCl}=0,15(mol)$
$\Rightarrow m_{CaCl_2}=0,15.111=16,65(g)$
$m_{Ca(OH)_2(dư)}=0,05.74=3,7(g)$
$2)$
$a)PTHH:Fe_2O_3+3CO\xrightarrow{t^o}2Fe+3CO_2\uparrow$
$b)n_{Fe}=\dfrac{22,4}{56}=0,4(mol)$
Theo PT: $n_{Fe_2O_3}=\dfrac{1}{2}n_{Fe}=0,2(mol)$
$n_{CO}=\dfrac{3}{2}n_{Fe}=0,6(mol)$
$\Rightarrow m_{Fe_2O_3}=0,2.160=32(g)$
$V_{CO}=0,6.22,4=13,44(lít)$

a, \(Fe_2O_3+3H_2\underrightarrow{t^o}2Fe+3H_2O\)
b, \(n_{Fe}=\dfrac{24}{56}=\dfrac{3}{7}\left(mol\right)\)
Theo PT: \(n_{Fe_2O_3}=\dfrac{1}{2}n_{Fe}=\dfrac{3}{14}\left(mol\right)\Rightarrow m_{Fe_2O_3}=\dfrac{3}{14}.160=\dfrac{240}{7}\left(g\right)\)
c, Theo PT: \(n_{H_2}=\dfrac{3}{2}n_{Fe}=\dfrac{9}{14}\left(mol\right)\Rightarrow V_{H_2}=\dfrac{9}{14}.22,4=14,4\left(l\right)\)
\(n_{Fe}=\dfrac{m}{M}=\dfrac{24}{56}\approx0,43\left(mol\right)\\ a.PTHH:Fe_2O_3+3H_2\underrightarrow{t^o}2Fe+3H_2O\)
2 3 2 3
0,43 0,645 0,45 0,645
\(b.m_{Fe_2O_3}=n.M=0,43.\left(56.2+16.3\right)=68,8\left(g\right)\\ c.V_{H_2}=n.24,79=0,645.24,79=15,98955\left(l\right).\)

nFe3O4 = 2,32 : 232 = 0,1 (mol)
pthh : 3Fe + 2O2 -t--> Fe3O4 (phản ứng hóa hợp )(có 2 chất sinh ra 1 chất mới)
0,3 <-- 0,2 < ------------0,1(mol)
=> VO2 = 0,2 . 22,4 = 4,48 (l)
%mFe = 168 .100 / 232 = 72,4 %
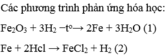
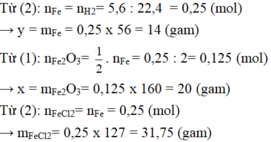
xem lại đề