Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a) \(n_{KMnO_4}=\dfrac{15,8}{158}=0,1\left(mol\right)\)
\(PTHH:2KMnO_4\underrightarrow{t^o}K_2MnO_4+MnO_2+O_2\uparrow\)
(mol)..........0,1................0,05..........0,05......0,05
\(V_{O_2}=0,05.22,4=1,12\left(l\right)\)
b) \(n_{Fe}=\dfrac{1.68}{56}=0,03\left(mol\right)\)
\(PTHH:3Fe+2O_2\underrightarrow{t^o}Fe_3O_4\)
(mol).......0,03....0,02.......0,1
\(PTHH:2KMnO_4\underrightarrow{t^o}K_2MnO_4+MnO_2+O_2\uparrow\)
(mol)..........0,04..............0,02............0,02....0,02
\(m_{KMnO_4}=0,04.158=6,32\left(g\right)\)
\(m_{KMnO_4\left(thựctế\right)}=6,32:95\%\approx6,65\left(g\right)\)

a) \(n_P=\dfrac{6,2}{31}=0,2\left(mol\right)\)
PTHH: 4P + 5O2 --to--> 2P2O5
0,2-->0,25
=> VO2 = 0,25.22,4 = 5,6 (l)
=> Vkk = 5,6.5 = 28 (l)
b)
PTHH: 2KMnO4 --to--> K2MnO4 + MnO2 + O2
0,5<-----------------------------0,25
=> \(m_{KMnO_4}=0,5.158=79\left(g\right)\)

Cảm ơn bạn @anayuiky đã nhắc lỗi sai. Mình sửa lại ý c):
PTHH: \(2KMnO_4\rightarrow^{t^o}K_2MnO_4+MnO_2+O_2\uparrow\)
Theo phương trình \(n_{KMnO_4}=n_{O_2}.2=0,25.2=0,5mol\)
\(\rightarrow m_{KMnO_4}=0,5.\left(39+55+16.4\right)=79g\)
a. \(n_{H_2}=\frac{V}{22,4}=\frac{11,2}{22,4}=0,5mol\)
\(n_{O_2}=\frac{V}{22,4}=\frac{10,08}{22,4}=0,45mol\)
PTHH: \(2H_2+O_2\rightarrow^{t^o}2H_2O\)
Ban đầu: 0,5 0,45 mol
Trong pứng: 0,5 0,25 0,5 mol
Sau pứng: 0 0,2 0,5 mol
\(\rightarrow M_{O_2\left(dư\right)}=n.M=0,2.32=6,4g\)
b. Theo phương trình \(n_{H_2O}=n_{H_2}=0,5mol\)
\(\rightarrow m_{H_2O}=n.M=0,5.18=9g\)
c. PTHH: \(2KMnO_4\rightarrow^{t^o}K_2MnO_4+MnO_2+O_2\uparrow\)
0,9 0,45 mol
\(\rightarrow n_{KMnO_4}=\frac{2}{1}n_{O_2}=\frac{0,45.2}{1}=0,9mol\)
\(\rightarrow m_{KMnO_4}=n.M=0,9.158=142,2g\)

\(n_{Ca}=\dfrac{0,4}{40}=0,01\left(mol\right)\)
PTHH: 2Ca + O2 --to--> 2CaO
0,01-->0,005
2KMnO4 --to--> K2MnO4 + MnO2 + O2
0,01<------------------------------0,005
=> \(m_{KMnO_4}=0,01.158=1,58\left(g\right)\)
\(n_{Ca}=\dfrac{0,4}{40}=0,01\left(mol\right)\)
PTHH: 2Ca + O2 --to--> 2CaO
0,01-->0,005
2KClO3 --to,MnO2--> 2KCl + 3O2
\(\dfrac{1}{300}\)<------------------------0,005
=> \(m_{KClO_3}=\dfrac{1}{300}.122,5=\dfrac{49}{120}\left(g\right)\)
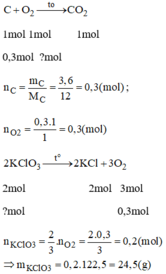


\(2KMnO4-->K2MnO4+MnO2+O2\)
\(C+O2-->CO2\)
\(n_C=\frac{4,8}{12}=0,4\left(mol\right)\)
\(n_{O2}=n_C=0,4\left(mol\right)\)
\(n_{KMNO4}=2n_{O2}=0,8\left(mol\right)\)
\(m_{KMnO4}=0,8.158=126,4\left(g\right)\)