Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

Gọi : \(\left\{{}\begin{matrix}n_{MgO}=a\left(mol\right)\\n_{Zn}=b\left(mol\right)\end{matrix}\right.\)⇒ 40a + 65b = 34(1)
\(MgO + 2HCl \to MgCl_2 + H_2O\\ Zn + 2HCl \to ZnCl_2 + H_2O\)
Muối gồm :\(\left\{{}\begin{matrix}n_{MgCl_2}=a\left(mol\right)\\n_{ZnCl_2}=b\left(mol\right)\end{matrix}\right.\)
Suy ra : 95a + 136b = 73,4(2)
Từ (1)(2) suy ra a = 0,2 ; b = 0,4
Vậy :
\(\%m_{MgO} = \dfrac{0,2.40}{34} .100\% = 23,53\%\\ \%m_{Zn} = 100\% - 23,53\% = 76,47\%\)

PTHH: MgO + 2 HCl -> MgCl2 + H2
x__________2x_______x______x(mol)
Zn + 2 HCl -> ZnCl2 + H2
y____2y_____y_____y(mol)
Ta có:
\(\left\{{}\begin{matrix}40x+65y=34\\95x+136y=73,4\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}x=0,2\\y=0,4\end{matrix}\right.\)
mMgO= 0,2.40=8(g)
=> %mMgO= \(\frac{8}{34}.100\approx23,529\%\)
=> %mZn \(\approx100\%-23,529\%\approx76,471\%\)

Gọi \(\left\{{}\begin{matrix}n_{CuO}=x\\n_{MgO}=y\end{matrix}\right.\)
\(CuO+2HCl\rightarrow CuCl_2+H_2O\)
x x ( mol )
\(MgO+2HCl\rightarrow MgCl_2+H_2O\)
y y ( mol )
Ta có:
\(\left\{{}\begin{matrix}80x+40y=16\\135x+95y=32,5\end{matrix}\right.\) \(\Leftrightarrow\left\{{}\begin{matrix}x=0,1\\y=0,2\end{matrix}\right.\)
\(\Rightarrow m_{CuO}=0,1.80=8g\)
\(\Rightarrow m_{MgO}=0,2.40=8g\)
\(\%m_{CuO}=\dfrac{8}{16}.100=50\%\)
\(\%m_{MgO}=\dfrac{8}{16}.100=50\%\)
\(m_{CuCl_2}=0,1.135=13,5g\)
\(m_{MgCl_2}=0,2.95=19g\)

a) Đặt \(\left\{{}\begin{matrix}n_{Al}=a\left(mol\right)\\n_{Fe}=b\left(mol\right)\end{matrix}\right.\) \(\Rightarrow27a+56b=11\) (1)
Ta có: \(n_{H_2}=\dfrac{8,96}{22,4}=0,4\left(mol\right)\)
Bảo toàn electron: \(3a+2b=0,4\cdot2=0,8\) (2)
Từ (1) và (2) \(\Rightarrow\left\{{}\begin{matrix}a=0,2\\b=0,1\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}\%m_{Al}=\dfrac{0,2\cdot27}{11}\cdot100\%\approx49,09\%\\\%m_{Fe}=50,91\%\end{matrix}\right.\)
b) Bảo toàn nguyên tố: \(\left\{{}\begin{matrix}n_{AlCl_3}=n_{Al}=0,2\left(mol\right)\\n_{FeCl_2}=n_{Fe}=0,1\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow m_{muối}=m_{AlCl_3}+m_{FeCl_2}=0,2\cdot133,5+0,1\cdot127=39,4\left(g\right)\)
c) Bảo toàn electron: \(3\cdot0,2+3\cdot0,1=2n_{SO_2}\)
\(\Rightarrow n_{SO_2}=0,45\left(mol\right)\) \(\Rightarrow V_{SO_2}=0,45\cdot22,4=10,08\left(l\right)\)
a) Gọi nAl = x, nFe = y
Có 27x + 56y = 11 (1)
Bảo toàn e
3x + 2y = 2.0,4 (2)
Từ 1 và 2 => x = 0,2, y = 0,1
\(\%mAl=\dfrac{0,2.27}{11}.100\%=49,09\%\)
\(\%mFe=100-49,09=50,91\%\)
b) BTKL:
m muối = mkim loại + mHCl - mH2
= 11 + 0,4.2.36,5 - 0,4.2 = 39,4g
c)
Bảo toàn e
Al => Al+3 + 3e S+6 + 2e => S+4
0,2 0,6 2x x
Fe => Fe+3 + 3e
0,1 0,3
=> 2x = 0,6 + 0,3 => x = 0,45 mol
=> VSO2 = 0,45.22,4 = 10,08 lít

Gọi số mol Fe2O3, CuO là a, b (mol)
nHCl = 0,3.2 = 0,6 (mol)
PTHH: Fe2O3 + 6HCl --> 2FeCl3 + 3H2O
a----->6a------->2a
CuO + 2HCl --> CuCl2 + H2O
b----->2b------->b
=> \(\left\{{}\begin{matrix}\dfrac{2a}{b}=\dfrac{3}{4}\\6a+2b=0,6\end{matrix}\right.\)
=> \(\left\{{}\begin{matrix}a=\dfrac{9}{170}\left(mol\right)\\b=\dfrac{12}{85}\left(mol\right)\end{matrix}\right.\)
=> \(\left\{{}\begin{matrix}\%m_{Fe_2O_3}=\dfrac{\dfrac{9}{170}.160}{\dfrac{9}{170}.160+\dfrac{12}{85}.80}.100\%=42,857\%\\\%m_{CuO}=\dfrac{\dfrac{12}{85}.80}{\dfrac{9}{170}.160+\dfrac{12}{85}.80}.100\%=57,143\%\end{matrix}\right.\)

\(n_{HCl}=\dfrac{200.14,6\%}{36,5}=0,8\left(mol\right)\\ Đặt:\left\{{}\begin{matrix}n_{Zn}=x\left(mol\right)\\n_{Mg}=y\left(mol\right)\end{matrix}\right.\\ Zn+2HCl\rightarrow ZnCl_2+H_2\\ Mg+2HCl\rightarrow MgCl_2+H_2\\ Tacó:\left\{{}\begin{matrix}65x+24y=12,5\\x+y=0,35\left(mol\right)\end{matrix}\right.\\ \Rightarrow\left\{{}\begin{matrix}x=0,1\\y=0,25\end{matrix}\right.\\ \Rightarrow m_{Zn}=6,5\left(g\right);m_{Mg}=6\left(g\right)\\ b.Tacó:BTNT\left(H\right):n_{HCl}.1>n_{H_2}.2\\ \Rightarrow HCldưsauphảnứng\\ Dungdịchsauphảnứnggồm:\left\{{}\begin{matrix}ZnCl_2:0,1\left(mol\right)\\MgCl_2:0,25\left(mol\right)\\HCl_{dư}:0,8-0,7=0,1\left(mol\right)\end{matrix}\right.\\ m_{ddsaupu}=200+12,5-0,35.2=212,8\left(g\right)\\ C\%_{ZnCl_2}=\dfrac{0,1.136}{212,8}.100=6,39\%;C\%_{MgCl_2}=\dfrac{0,25.95}{212,8}.100=11,16\%;C\%_{HCl\left(dư\right)}=\dfrac{0,1.36,5}{212,8}.100=1,72\%\)

PTHH: \(2Fe+6H_2SO_{4\left(đ\right)}\underrightarrow{t^o}Fe_2\left(SO_4\right)_3+3SO_2\uparrow+6H_2O\)
\(CuO+H_2SO_4\rightarrow CuSO_4+H_2O\)
a) Ta có: \(n_{SO_2}=\dfrac{2,24}{22,4}=0,1\left(mol\right)\) \(\Rightarrow n_{Fe}=\dfrac{1}{15}\left(mol\right)\)
\(\Rightarrow\%m_{Fe}=\dfrac{\dfrac{1}{15}\cdot56}{13,6}\cdot100\%\approx27,45\%\) \(\Rightarrow\%m_{CuO}=72,55\%\)
b) Ta có: \(m_{CuO}=13,6-\dfrac{1}{15}\cdot56\approx9,9\left(g\right)\) \(\Rightarrow n_{CuO}=n_{H_2SO_4}=\dfrac{9,9}{80}=0,12375\left(mol\right)\)
*Làm gì có H2SO4 loãng đâu nhỉ ??

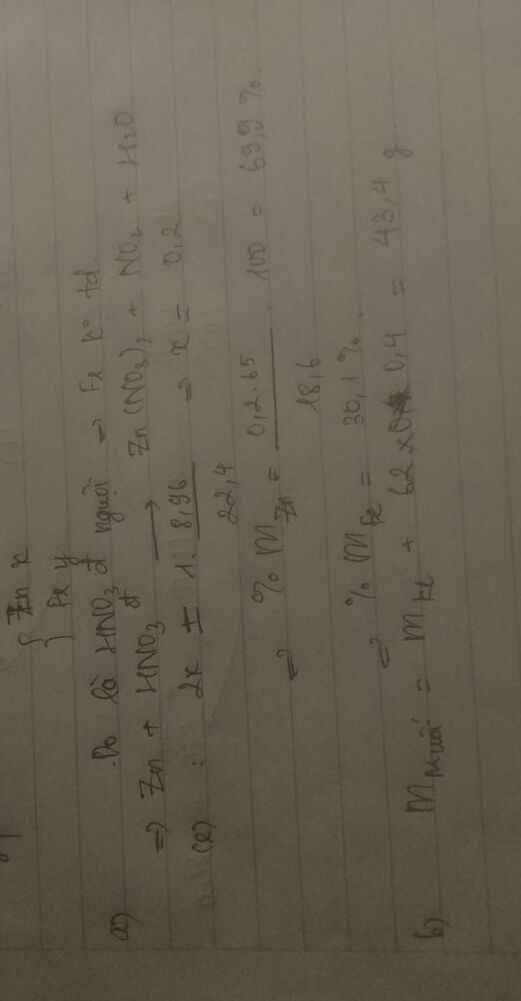

Gọi số mol của MgO và Zn là a và b
=> 40a + 65b = 34
PTHH: MgO + 2HCl --> MgCl2 + H2O
______a------------------->a
Zn + 2HCl --> ZnCl2 + H2
b---------------->b
=> 95a + 136b = 73,4
=> a = 0,2; b = 0,4
=> \(\left\{{}\begin{matrix}\%MgO=\dfrac{0,2.40}{34}.100\%=23,529\%\\\%Zn=\dfrac{0,4.65}{34}.100\%=76,471\%\end{matrix}\right.\)