
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


a) (1) Fe + 2FeCl3 → 3FeCl2
(2) 3Ba(OH)2 + 2FeCl3 → 3BaCl2+ 2Fe(OH)3↓
(3) 2Fe(OH)3 + 3H2SO4 → Fe2(SO4)3 + 6H2O
(4) Fe2(SO4)3 + 3BaCl2 → 2FeCl3+ 3BaSO4↓
b) (1) 3NaOH+Fe(NO3)3→3NaNO3+Fe(OH)3↓
(2)2Fe(OH)3 t0 → Fe2O3+3H2O
(3)2Al + Fe2O3 → Al2O3+2Fe
(4)Fe + 2HCl→FeCl2+H2↑
(5) FeCl2 + 2NaOH → 2NaCl + Fe(OH)2

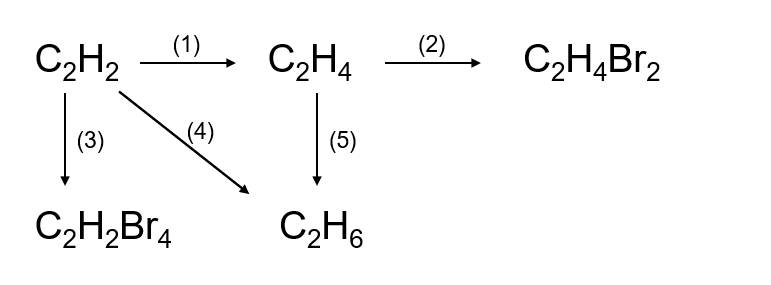
(1) \(2CH_4\xrightarrow[lln]{1500^oC}C_2H_2+3H_2\)
(2) \(C_2H_2+H_2\underrightarrow{t^o,Pd}C_2H_4\)
(3) \(C_2H_4+Br_2\rightarrow C_2H_4Br_2\)
(4) \(CH_4+Cl_2\underrightarrow{as}CH_3Cl+HCl\)
(5) \(C_2H_2+2Br_2\rightarrow C_2H_2Br_4\)
(6) \(C_2H_2+2H_2\underrightarrow{t^o,Ni}C_2H_6\)
(7) \(C_2H_4+H_2\underrightarrow{t^o,Ni}C_2H_6\)
(1) 2��4→���1500���2�2+3�22CH41500oCllnC2H2+3H2
(2) �2�2+�2��,��→�2�4C2H2+H2to,PdC2H4
(3) �2�4+��2→�2�4��2C2H4+Br2→C2H4Br2
(4) ��4+��2��→��3��+���CH4+Cl2asCH3Cl+HCl
(5) �2�2+2��2→�2�2��4C2H2+2Br2→C2H2Br4
(6) �2�2+2�2��,��→�2�6C2H2+2H2to,NiC2H6
(7) �2�4+�2��,��→�2�6C2H4+H2to,NiC2H6

A : CuO
B : C
C : CO2
D : Ca(OH)2
PTHH: 2CuO + C ---to→ 2Cu + CO2
CO2 + Ca(OH)2 → CaCO3 + H2O
A là CuO
B là C (trong dạng than)
C là CO2 và có CO
D là NaOH
PTHH: CuO+C=>Cu+CO2+CO
NaOH+CO2=>NaHCO3+H2O
NaOH+CO2=>Na2Co3+H2O

- Mg + 2HCl ===> MgCl2 + H2
- MgCl2 + Ba(OH)2 ===> Mg(OH)2 + BaCl2
- Mg(OH)2 =(nhiệt)==> MgO + H2O
- MgO + H2SO4===> MgSO4 + H2O
- MgSO4 + BaCl2 ===> MgCl2 + BaSO4

Câu 3:
N2+O2\(\overset{t^0}{\rightarrow}\)2NO
4NO+3O2+2H2O\(\rightarrow\)4HNO3
NO3- : làm tăng lượng phân đạm cho cây!

(1) C + O 2 → t ° C O 2
(2) C O 2 + Ca OH 2 → CaC O 3 + H 2 O
(3) CaC O 3 → CaO + C O 2
(4) CaO + H 2 O → Ca OH 2
(5) Ca OH 2 + 2C O 2 → Ca HCO 3 2

(1) Fe 2 O 3 + 3 H 2 → 2Fe + 3 H 2 O
(2) 2Fe + 3 Cl 2 → 2Fe Cl 3
(3) Fe Cl 3 + 3NaOH → 3NaCl + Fe OH 3
(4) 2Fe OH 3 → Fe 2 O 3 + 3 H 2 O
(5) Fe + HCl → Fe Cl 2 + H 2
(6) Fe Cl 2 + 2NaOH → Fe OH 2 + 2NaCl

PTHH:
- 2Al + 6HCl ===> 2AlCl3 + 3H2
- AlCl3 + 3AgNO3 ===> Al(NO3)3 + 3AgCl
- Al(NO3)3 + 3NaOH ===> Al(OH)3 + 3NaNO3
- 2Al(OH)3 =(nhiệt)=> Al2O3 + 3H2O
- Al2O3 + 3H2SO4 ===> Al2(SO4)3 + 3H2O
Al \(\underrightarrow{\left(1\right)}\) AlCl3 \(\underrightarrow{\left(2\right)}\) Al(NO3)3 \(\underrightarrow{\left(3\right)}\) Al(OH)3 \(\underrightarrow{\left(4\right)}\) Al2O3 \(\underrightarrow{\left(5\right)}\) Al2(SO4)3
PTHH
(1) 2Al + 6HCl \(\rightarrow\) 2AlCl3 + 3H2
(2) AlCl3 + 3AgNO3 \(\rightarrow\) 3AgCl \(\downarrow\) + Al(NO3)3
(3) Al(NO3)3 + 3NaOH \(\rightarrow\) 3NaNO3 + Al(OH)3 \(\downarrow\)
(4) 4Al(OH)3 \(\underrightarrow{t^0}\) 2Al2O3 + 6H2O
(5) Al2O3 + 3H2SO4 \(\rightarrow\) Al2(SO4)3 + 3H2O

\(Na_2O+H_2O\rightarrow2NaOH\\ 2NaOH+CO_2\rightarrow Na_2CO_3+H_2O\\ Na_2CO_3+2HCl\rightarrow2NaCl+CO_2+H_2O\\ CO_2+H_2O\rightarrow H_2CO_3\)
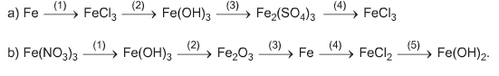












 Giúp mình giải vài câu thực tế Hoá nha..
Giúp mình giải vài câu thực tế Hoá nha..





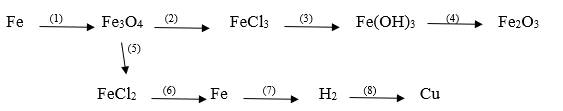
(1) \(3Fe+2O_2\xrightarrow[]{t^\circ}Fe_3O_4\)
(2) \(Fe_3O_4+8HCl\xrightarrow[]{}FeCl_2+2FeCl_3+4H_2O\)
(3) \(FeCl_3+3LiOH\xrightarrow[]{}Fe\left(OH\right)_3\downarrow+3LiCl\)
(4) \(2Fe\left(OH\right)_3\xrightarrow[]{t^\circ}Fe_2O_3+3H_2O\)
(5) \(Fe_3O_4+8HCl\xrightarrow[]{}FeCl_2+2FeCl_3+4H_2O\)
(6) \(FeCl_2\xrightarrow[]{đpdd}Fe+Cl_2\uparrow\)
(7) \(Fe+2HCl\xrightarrow[]{}FeCl_2+H_2\uparrow\)
(8) \(H_2+CuO\xrightarrow[]{t^\circ}Cu+H_2O\uparrow\)