Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(C\%_{KCl}=\dfrac{20}{600}\cdot100\%=3.33\%\)
\(C\%_{K_2SO_4}=\dfrac{75}{1500}\cdot100\%=5\%\)
\(C\%_{NaCl}=\dfrac{15}{15+45}\cdot100\%=25\%\)

\(C\)\(\%\)\(=\dfrac{m_{ct}}{m_{dd}} .100\)\(\%\)= \(\dfrac{20}{500} . 100\)\(\%\)\(=4 \)\(\%\)



Bài 1:
\(n_{KNO_3}=\dfrac{20}{101}=0,198\left(mol\right)\)
\(C_M=\dfrac{n}{V}=\dfrac{0,198}{0,85}=0,233M\)
Bài 2:
\(C_M=\dfrac{n}{V}=\dfrac{0,5}{0,75}=0,66M\)
Bài 3:
\(n_{KNO_3}=2.0,5=1\left(mol\right)\)
\(m_{KNO_3}=1.101=101\left(g\right)\)
Bài 4:
\(C\%=\dfrac{20}{600}.100=3,33\%\)
Bài 1:
\(n_{KNO_3}=\dfrac{20}{101}=0,198\left(mol\right)\)
\(C_{M_{ddKNO_3}}=\dfrac{0,198}{0,85}\approx0,23M\)
Bài 2:
\(C_{M_{ddKCl}}=\dfrac{0,5}{0,75}\approx0,667M\)
Bài 3:
\(n_{KNO_3}=0,5.2=1\left(mol\right)\Rightarrow m_{KNO_3}=1.101=101\left(g\right)\)
Bài 4:
\(C\%_{ddKCl}=\dfrac{20.100\%}{600}=3,333\%\)

a) \(C_{M_{MgCl_2}}=\frac{0,5}{0,75}=0,667\left(M\right)\)
b) \(n_{CuSO_4}=\frac{400}{160}=2,5\left(mol\right)\)
\(\Rightarrow C_{M_{CuSO_4}}=\frac{2,5}{4}=0,625\left(M\right)\)
c) \(C\%_{KCl}=\frac{20}{600}\times100\%=3,33\%\)
d) \(m_{ddNaCl}=20+180=200\left(g\right)\)
\(C\%_{NaCl}=\frac{20}{200}\times100\%=10\%\)
e) \(n_{KNO_3}=0,5\times2=1\left(mol\right)\)
\(\Rightarrow m_{KNO_3}=1\times101=101\left(g\right)\)
f) \(m_{MgCl_2}=50\times4\%=2\left(g\right)\)
\(n_{MgCl_2}=\frac{2}{95}\left(mol\right)\)
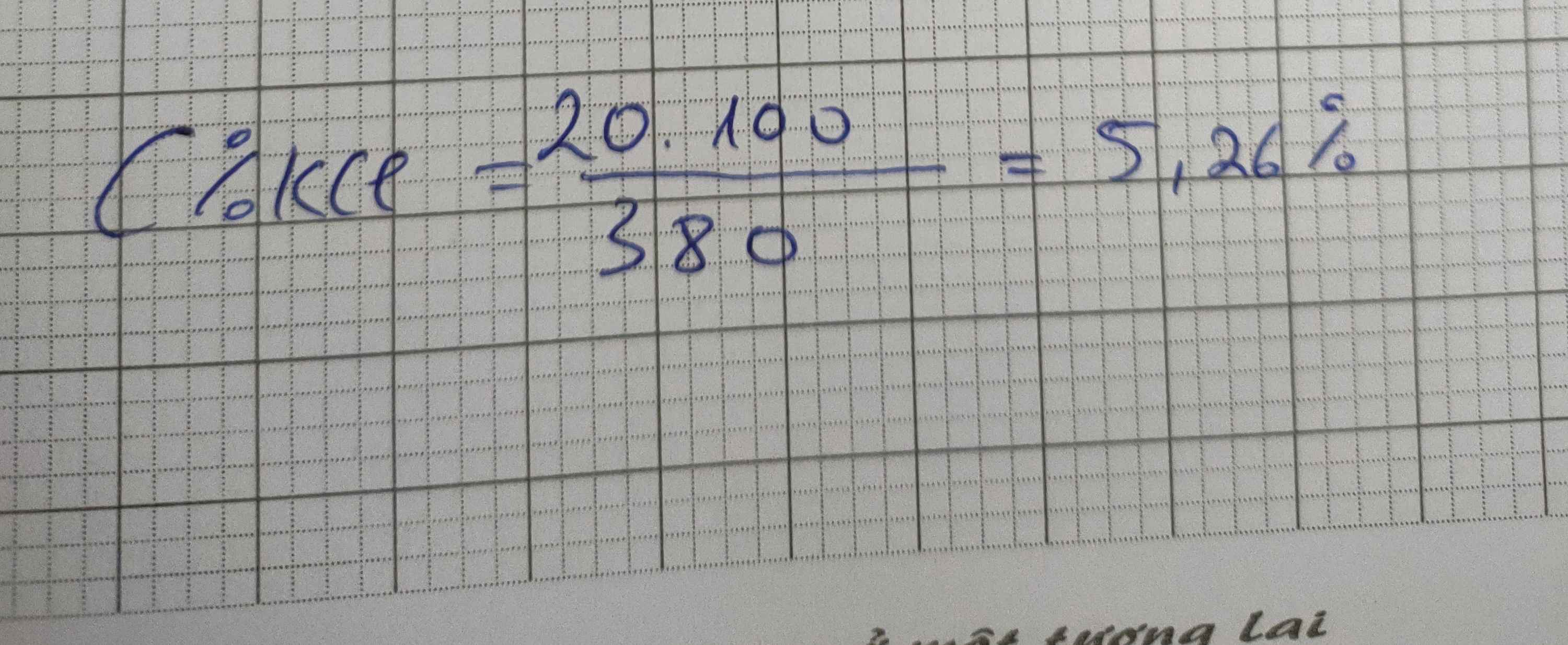
a. \(C\%=\frac{m_{ct}}{m_{dd}}100\%=\frac{20}{180+20}.100\%=10\%\)
b. \(C\%=\frac{30}{150}100\%=20\%\)
c. \(C\%=\frac{20}{400}100\%=5\%\)
A/ C%NaCl= 20/200 * 100%= 10%
B/ C%H2SO4= 30/150 * 100%= 20%
C/ C%KCl= 20/400 * 100%5= 5%