Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

Bài 1
H2+ 1/2O2 --> H2O
Mg + 1/2O2 --> MgO
Cu+ 1/2O2-->CuO
S+O2 -->SO2
4Al+ 3O2-->2Al2O3
C+ O2--> CO2
2P+5/2O2--> P2O5
Bài 2
CH4+2O2->CO2+2H2O
2C2H2+5O2->4CO2+2H2O
C2H6O+3O2->2CO2+3H2O

\(1,2H_2+O_2\underrightarrow{t}2H_2O\)
\(2Mg+O_2\underrightarrow{t}2MgO\)
\(2Cu+O_2\underrightarrow{t}2CuO\)
\(S+O_2\underrightarrow{t}SO_2\)
\(4Al+3O_2\underrightarrow{t}2Al_2O_3\)
\(C+O_2\underrightarrow{t}CO_2\)
\(4P+5O_2\underrightarrow{t}2P_2O_5\)
\(2,PTHH:C+O_2\underrightarrow{t}CO_2\)
\(a,n_{O_2}=0,2\left(mol\right)\Rightarrow n_{CO_2}=0,2\left(mol\right)\Rightarrow m_{CO_2}=8,8\left(g\right)\)
\(b,n_C=0,3\left(mol\right)\Rightarrow n_{CO_2}=0,3\left(mol\right)\Rightarrow m_{CO_2}=13,2\left(g\right)\)
c, Vì\(\frac{0,3}{1}>\frac{0,2}{1}\)nên C phản ửng dư, O2 phản ứng hết, Bài toán tính theo O2
\(n_{O_2}=0,2\left(mol\right)\Rightarrow n_{CO_2}=0,2\left(mol\right)\Rightarrow m_{CO_2}=8,8\left(g\right)\)
\(3,PTHH:CH_4+2O_2\underrightarrow{t}CO_2+2H_2O\)
\(C_2H_2+\frac{5}{2}O_2\underrightarrow{t}2CO_2+H_2O\)
\(C_2H_6O+3O_2\underrightarrow{t}2CO_2+3H_2O\)
\(4,a,PTHH:4P+5O_2\underrightarrow{t}2P_2O_5\)
\(n_P=1,5\left(mol\right)\Rightarrow n_{O_2}=1,2\left(mol\right)\Rightarrow m_{O_2}=38,4\left(g\right)\)
\(b,PTHH:C+O_2\underrightarrow{t}CO_2\)
\(n_C=2,5\left(mol\right)\Rightarrow n_{O_2}=2,5\left(mol\right)\Rightarrow m_{O_2}=80\left(g\right)\)
\(c,PTHH:4Al+3O_2\underrightarrow{t}2Al_2O_3\)
\(n_{Al}=2,5\left(mol\right)\Rightarrow n_{O_2}=1,875\left(mol\right)\Rightarrow m_{O_2}=60\left(g\right)\)
\(d,PTHH:2H_2+O_2\underrightarrow{t}2H_2O\)
\(TH_1:\left(đktc\right)n_{H_2}=1,5\left(mol\right)\Rightarrow n_{O_2}=0,75\left(mol\right)\Rightarrow m_{O_2}=24\left(g\right)\)
\(TH_2:\left(đkt\right)n_{H_2}=1,4\left(mol\right)\Rightarrow n_{O_2}=0,7\left(mol\right)\Rightarrow m_{O_2}=22,4\left(g\right)\)
\(5,PTHH:S+O_2\underrightarrow{t}SO_2\)
\(n_{O_2}=0,46875\left(mol\right)\)
\(n_{SO_2}=0,3\left(mol\right)\)
Vì\(0,46875>0,3\left(n_{O_2}>n_{SO_2}\right)\)nên S phản ứng hết, bài toán tính theo S.
\(a,\Rightarrow n_S=n_{SO_2}=0,3\left(mol\right)\Rightarrow m_S=9,6\left(g\right)\)
\(n_{O_2}\left(dư\right)=0,16875\left(mol\right)\Rightarrow m_{O_2}\left(dư\right)=5,4\left(g\right)\)
\(6,a,PTHH:C+O_2\underrightarrow{t}CO_2\)
\(n_{O_2}=1,5\left(mol\right)\Rightarrow n_C=1,5\left(mol\right)\Rightarrow m_C=18\left(g\right)\)
\(b,PTHH:2H_2+O_2\underrightarrow{t}2H_2O\)
\(n_{O_2}=1,5\left(mol\right)\Rightarrow n_{H_2}=0,75\left(mol\right)\Rightarrow m_{H_2}=1,5\left(g\right)\)
\(c,PTHH:S+O_2\underrightarrow{t}SO_2\)
\(n_{O_2}=1,5\left(mol\right)\Rightarrow n_S=1,5\left(mol\right)\Rightarrow m_S=48\left(g\right)\)
\(d,PTHH:4P+5O_2\underrightarrow{t}2P_2O_5\)
\(n_{O_2}=1,5\left(mol\right)\Rightarrow n_P=1,2\left(mol\right)\Rightarrow m_P=37,2\left(g\right)\)
\(7,n_{O_2}=5\left(mol\right)\Rightarrow V_{O_2}=112\left(l\right)\left(đktc\right)\);\(V_{O_2}=120\left(l\right)\left(đkt\right)\)
\(8,PTHH:C+O_2\underrightarrow{t}CO_2\)
\(m_C=0,96\left(kg\right)\Rightarrow n_C=0,08\left(kmol\right)=80\left(mol\right)\Rightarrow n_{O_2}=80\left(mol\right)\Rightarrow V_{O_2}=1792\left(l\right)\)
\(9,n_p=0,2\left(mol\right);n_{O_2}=0,3\left(mol\right)\)
\(PTHH:4P+5O_2\underrightarrow{t}2P_2O_5\)
Vì\(\frac{0,2}{4}< \frac{0,3}{5}\)nên P hết O2 dư, bài toán tính theo P.
\(a,n_{O_2}\left(dư\right)=0,05\left(mol\right)\Rightarrow m_{O_2}\left(dư\right)=1,6\left(g\right)\)
\(b,n_{P_2O_5}=0,1\left(mol\right)\Rightarrow m_{P_2O_5}=14,2\left(g\right)\)

\(n_{O_2}=\dfrac{1456:1000}{22,4}=0,065\left(mol\right)\\ a,2C_4H_{10}+13O_2\rightarrow\left(t^o\right)8CO_2+10H_2O\\ b,n_{CO_2}=\dfrac{8}{13}.0,065=0,04\left(mol\right)\\ n_{H_2O}=\dfrac{10}{13}.0,065=0,05\left(mol\right)\\ b,m_{sp}=m_{CO_2}+m_{H_2O}=44.0,04+18.0,05=2,66\left(g\right)\\ c,n_{C_4H_{10}}=\dfrac{2}{13}.0,065=0,01\left(mol\right)\\ V_{gas}=\dfrac{100}{80}.0,01.22,4=0,28\left(l\right)\)

Bài 1:
a, PT: \(Na_2O+H_2O\rightarrow2NaOH\)
b, Ta có: \(n_{Na_2O}=\dfrac{31}{62}=0,5\left(mol\right)\)
\(n_{H_2O}=\dfrac{27}{18}=1,5\left(mol\right)\)
Xét tỉ lệ: \(\dfrac{0,5}{1}< \dfrac{1,5}{1}\), ta được H2O dư.
Theo PT: \(n_{NaOH}=2n_{Na_2O}=1\left(mol\right)\)
\(\Rightarrow m_{NaOH}=1.40=40\left(g\right)\)
b, Theo PT: \(n_{H_2O\left(pư\right)}=n_{Na_2O}=0,5\left(mol\right)\)
\(\Rightarrow n_{H_2O\left(dư\right)}=1,5-0,5=1\left(mol\right)\)
\(\Rightarrow m_{H_2O\left(dư\right)}=1.18=18\left(g\right)\)
Bài 2:
a, PT: \(CH_4+2O_2\underrightarrow{t^o}CO_2+2H_2O\)
Ta có: \(n_{CH_4}=\dfrac{2,24}{22,4}=0,1\left(mol\right)\)
\(n_{O_2}=\dfrac{3,36}{22,4}=0,15\left(mol\right)\)
b, Xét tỉ lệ: \(\dfrac{0,1}{1}>\dfrac{0,15}{2}\), ta được CH4 dư.
Theo PT: \(n_{CH_4\left(pư\right)}=\dfrac{1}{2}n_{O_2}=0,075\left(mol\right)\)
\(\Rightarrow n_{CH_4\left(dư\right)}=0,1-0,075=0,025\left(mol\right)\)
\(\Rightarrow V_{CH_4\left(dư\right)}=0,025.22,4=0,56\left(l\right)\)
c, Theo PT: \(\left\{{}\begin{matrix}n_{CO_2}=\dfrac{1}{2}n_{O_2}=0,075\left(mol\right)\\n_{H_2O}=n_{O_2}=0,15\left(mol\right)\end{matrix}\right.\)
⇒ m sản phẩm = mCO2 + mH2O = 0,075.44 + 0,15.18 = 6 (g)

Bài 1:
a) 2CuFeS2 + \(\dfrac{13}{2}\)O2 --to--> 2CuO + Fe2O3 + 4SO2
b) \(n_{CuFeS_2}=\dfrac{3,68}{184}=0,02\left(mol\right)\)
\(n_{O_2}=\dfrac{1,68}{22,4}=0,075\left(mol\right)\)
Xét tỉ lệ: \(\dfrac{0,02}{2}< \dfrac{0,075}{\dfrac{13}{2}}\) => CuFeS2 hết, O2 dư
PTHH: 2CuFeS2 + \(\dfrac{13}{2}\)O2 --to--> 2CuO + Fe2O3 + 4SO2
0,02----->0,065------->0,02---->0,01---->0,04
=> \(\left\{{}\begin{matrix}n_{O_2\left(dư\right)}=0,075-0,065=0,01\left(mol\right)\\n_{SO_2}=0,04\left(mol\right)\end{matrix}\right.\)
=> \(\left\{{}\begin{matrix}\%V_{O_2}=\dfrac{0,01}{0,01+0,04}.100\%=20\%\\\%V_{SO_2}=\dfrac{0,04}{0,01+0,04}.100\%=80\%\end{matrix}\right.\)
- \(\left\{{}\begin{matrix}m_{CuO}=0,02.80=1,6\left(g\right)\\m_{Fe_2O_3}=0,01.160=1,6\left(g\right)\end{matrix}\right.\)
=> mrắn = 1,6 + 1,6 = 3,2 (g)
Bài 2:
a)
2CuS + 3O2 --to--> 2CuO + 2SO2
4FeS + 7O2 --to--> 2Fe2O3 + 4SO2
b) Gọi số mol CuS, FeS là a, b (mol)
=> 96a + 88b = 22,8 (1)
\(n_{SO_2}=\dfrac{5,6}{22,4}=0,25\left(mol\right)\)
=> a + b = 0,25 (2)
(1)(2) => a = 0,1; b = 0,15
=> \(\left\{{}\begin{matrix}n_{CuO}=0,1\left(mol\right)\\n_{Fe_2O_3}=0,075\left(mol\right)\end{matrix}\right.\)
=> \(\left\{{}\begin{matrix}\%m_{CuO}=\dfrac{0,1.80}{0,1.80+0,075.160}.100\%=40\%\\\%m_{Fe_2O_3}=\dfrac{0,075.160}{0,1.80+0,075.160}.100\%=60\%\end{matrix}\right.\)

a, Theo giả thiết ta có: \(n_P=\dfrac{3,1}{31}=0,1\left(mol\right)\)
\(4P+5O_2--t^o->2P_2O_5\)
Ta có: \(n_{O_2}=\dfrac{5}{4}.n_P=0,125\left(mol\right)\Rightarrow V_{O_2\left(đktc\right)}=0,125.22,4=2,8\left(l\right)\)
b, Theo giả thiết ta có: \(n_{CH_4}=\dfrac{1,12}{22,4}=0,05\left(mol\right)\)
\(CH_4+2O_2--t^o->CO_2+2H_2O\)
Ta có: \(n_{O_2}=2.n_{CH_4}=0,1\left(mol\right)\Rightarrow V_{O_2\left(đktc\right)}=2,24\left(l\right)\)

a) PTHH: \(2Cu+O_2\xrightarrow[]{t^o}2CuO\)
b) \(n_{Cu}=\dfrac{m_{Cu}}{M_{Cu}}=\dfrac{38,4}{64}=0,6\left(mol\right)\)
Theo PTHH: \(n_{O_2}=\dfrac{1}{2}n_{Cu}\)
⇒ \(n_{O_2}=\dfrac{1}{2}.0,6=0,3\left(mol\right)\)
\(\Rightarrow V_{O_2}=n_{O_2}.22,4=0,3.22,4=6,72\left(l\right)\)
c) \(CuO+H_2\xrightarrow[]{t^o}Cu+H_2O\)
\(n_{H_2}=\dfrac{11,2}{22,4}=0,5\left(mol\right)\)
Theo PTHH: \(n_{H_2}=n_{Cu}=n_{H_2O}=0,5\left(mol\right)\)
\(\Rightarrow m_{H_2O}=n_{H_2O}.M_{H_2O}=0,5.18=9\left(g\right)\)
\(\Rightarrow m_{Cu}=n_{Cu}.M_{Cu}=0,5.64=32\left(g\right)\)
a) PTHH: \(2Cu+O_2\xrightarrow[]{t^o}2CuO\)
b) \(n_{Cu}=\dfrac{m_{Cu}}{M_{Cu}}=\dfrac{38,4}{64}=0,6\left(mol\right)\)
Theo PTHH: \(n_{O_2}=\dfrac{1}{2}n_{Cu}\)
\(\Rightarrow n_{O_2}=0,3\left(mol\right)\)
\(\Rightarrow V_{O_2}=0,3.22,4=6,72\left(l\right)\)
c) Theo PTHH : \(n_{CuO}=n_{Cu}=0,6\left(mol\right)\)
Khối lượng đồng oxit thu được sau phản ứng:
\(\Rightarrow m_{CuO}=0,3.80=24\left(g\right)\)
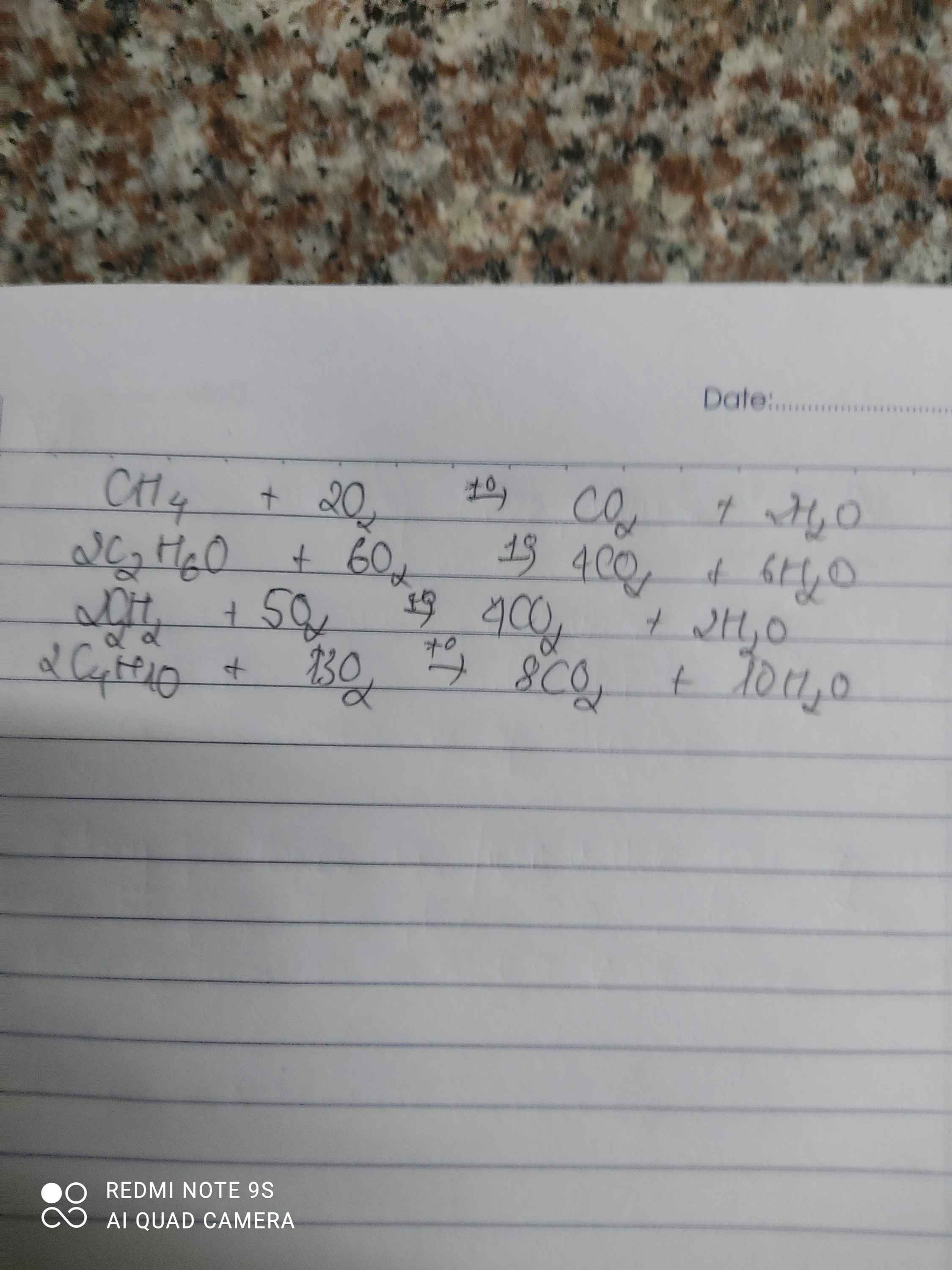
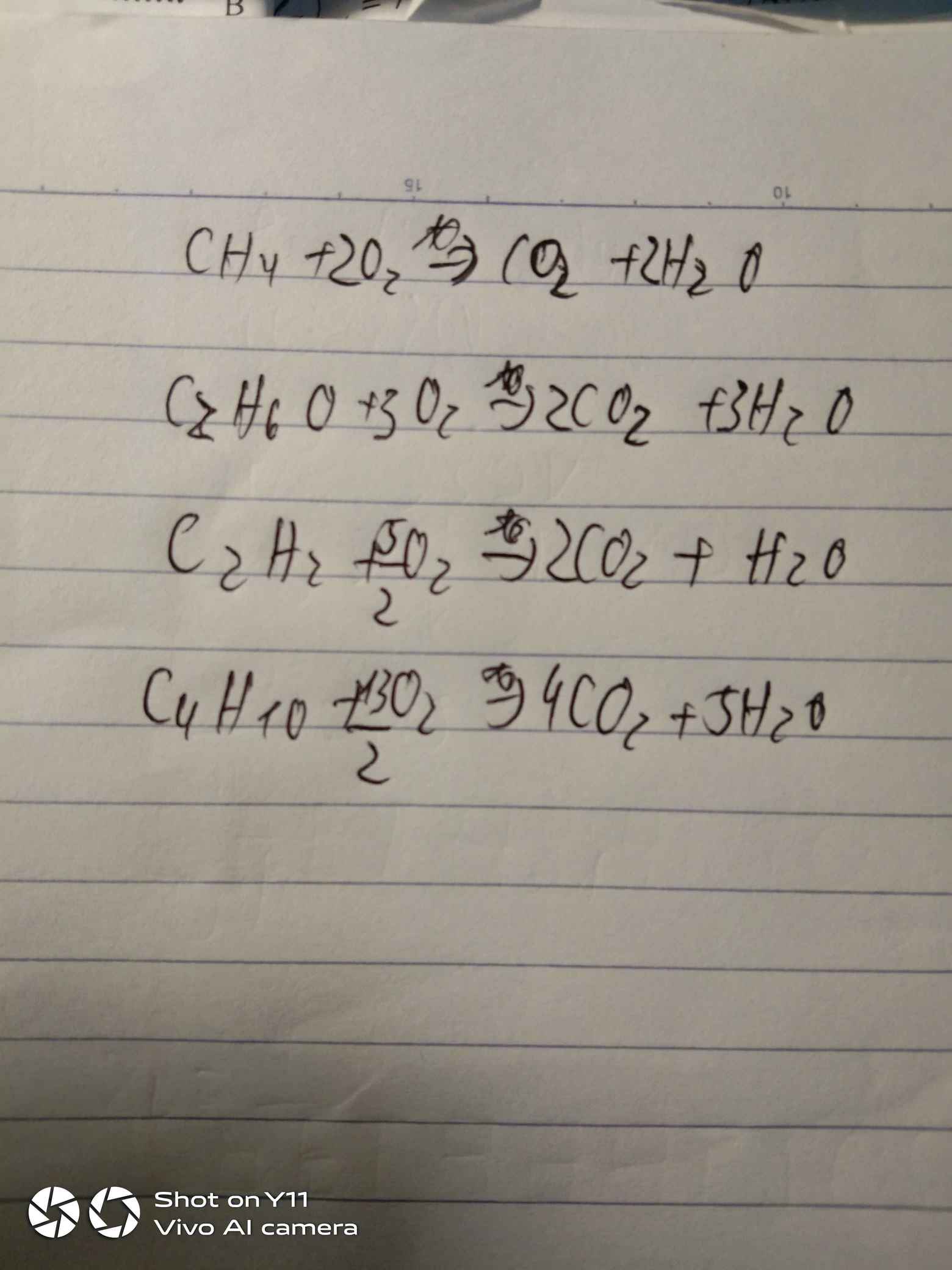

a)
\(CH_4+2O_2\rightarrow CO_2+H_2O\)
\(2C_2H_2+5O_2\rightarrow4CO_2+2H_2O\)
\(C_2H_5OH+3O_2\rightarrow2CO_2+3H_2O\)
b)
CH4: \(n_{CO2}=0,5\left(mol\right),n_{H2O}=1\left(mol\right)\)
\(m=0,5.44+1.18=40\left(g\right)\)
C2H2:
\(n_{CO2}=1\left(mol\right),n_{H2O}=0,5\left(mol\right)\)
\(m=1.44+0,5.18=53\left(g\right)\)
C2H5OH
\(n_{CO2}=1\left(mol\right),n_{H2O}=1,5\left(mol\right)\)
\(m=1.44+1,5.18=71\left(g\right)\)
làm chung câu a và b cho dễ hiểu nhé
CH4+2O2--->CO2+2H2O
0,5------------0,5-----1(mol)
m CO2=0,5.44=22(g)
m H2O=1/18=18(g)
2C2H2+5O2--->4CO2+2H2O
0,5----------------1---------0,5(mol)
m CO2=1.44=44(g)
m H2O=0,5.18=9(g)
2C2H5OH+7O2----->4CO2+6H2O
0,5------------------------1--------1,5(mol)
m CO2=1.44=44(g)
m H2O=1.5.18=27(g)