Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

Bài 9:
\(n_{Zn}=\dfrac{6,5}{65}=0,1\left(mol\right)\\ n_{HCl}=\dfrac{14,6\%.100}{36,5}=0,4\left(mol\right)\\ PTHH:Zn+2HCl\rightarrow ZnCl_2+H_2\\ Vì:\dfrac{0,1}{1}< \dfrac{0,4}{2}\Rightarrow HCldư\\ n_{HCl\left(dư\right)}=0,4-2.0,1=0,2\left(mol\right)\\ m_{HCl\left(dư\right)}=0,2.36,5=7,3\left(g\right)\)

\(n_{CuSO_4}=\dfrac{50}{250}=0.2\left(mol\right)\)
\(n_{FeSO_4}=\dfrac{27.8}{278}=0.1\left(mol\right)\)
\(C_{M_{CuSO_4}}=C_{M_{FeSO_4}}=\dfrac{0.1}{0.1964}=0.5\left(M\right)\)
\(m_{dd_A}=50+27.8+196.4=274.2\left(g\right)\)
\(C\%_{CuSO_4}=\dfrac{0.1\cdot160}{274.2}\cdot100\%=6.47\%\)
\(C\%_{FeSO_4}=\dfrac{0.1\cdot152}{274.2}\cdot100\%=5.54\%\)
\(n_{CuSO_4.5H_2O}=\dfrac{50}{250}=0,2\left(mol\right)\)
=> \(m_{CuSO_4}=0,2.160=32\left(g\right)\)
\(m_{H_2O}=0,2.5.18=18\left(g\right)\)
\(n_{FeSO_4.7H_2O}=\dfrac{27,8}{278}=0,1\left(mol\right)\)=> \(m_{FeSO_4}=0,1.152=15,2\left(g\right)\)
\(m_{H_2O}=0,1.7.18=12,6\left(g\right)\)
\(m_{dd}=196,4+50+27,8=274,2\left(g\right)\)
\(V_{dd}=\dfrac{196,4+18+12,6}{1000}=0,227\left(l\right)\)
=> \(CM_{CuSO_4}=\dfrac{0,2}{0,227}=0,72M\)
\(C\%_{CuSO_4}=\dfrac{32}{274,2}.100=11,67\%\)
\(CM_{FeSO_4}=\dfrac{0,1}{0,227}=0,44M\)
\(C\%_{CuSO_4}=\dfrac{15,2}{274,2}.100=5,54\%\)

a) PTHH: \(MgO+2HCl\rightarrow MgCl_2+H_2O\)
b) Ta có: \(n_{MgO}=\dfrac{8}{40}=0,2\left(mol\right)\)
\(\Rightarrow\left\{{}\begin{matrix}n_{MgCl_2}=0,2\left(mol\right)\\n_{HCl}=0,4\left(mol\right)\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}m_{MgCl_2}=0,2\cdot95=19\left(g\right)\\C\%_{HCl}=\dfrac{0,4\cdot36,5}{200}\cdot100\%=7,3\%\end{matrix}\right.\)
Mặt khác: \(m_{dd\left(sau.p/ứ\right)}=m_{MgO}+m_{ddHCl}=208\left(g\right)\)
\(\Rightarrow C\%_{MgCl_2}=\dfrac{19}{208}\cdot100\%\approx9,13\%\)
c) PTHH: \(MgCl_2+2NaOH\rightarrow2NaCl+Mg\left(OH\right)_2\downarrow\)
Ta có: \(\left\{{}\begin{matrix}n_{MgCl_2}=0,2\left(mol\right)\\n_{NaOH}=\dfrac{200\cdot4\%}{40}=0,2\left(mol\right)\end{matrix}\right.\)
Xét tỉ lệ: \(\dfrac{0,2}{1}>\dfrac{0,2}{2}\) \(\Rightarrow\) NaOH p/ứ hết, MgCl2 còn dư
\(\Rightarrow\left\{{}\begin{matrix}n_{NaCl}=0,2\left(mol\right)\\n_{Mg\left(OH\right)_2}=0,1\left(mol\right)=n_{MgCl_2\left(dư\right)}\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}m_{NaCl}=0,2\cdot58,5=11,7\left(g\right)\\m_{MgCl_2\left(dư\right)}=9,5\left(g\right)\\m_{Mg\left(OH\right)_2}=0,1\cdot58=5,8\left(g\right)\end{matrix}\right.\)
Mặt khác: \(m_{dd\left(sau.p/ứ\right)}=m_{ddA}+m_{ddNaOH}-m_{Mg\left(OH\right)_2}=402,2\left(g\right)\)
\(\Rightarrow\left\{{}\begin{matrix}C\%_{NaCl}=\dfrac{11,7}{402,2}\cdot100\%\approx2,91\%\\C\%_{MgCl_2\left(dư\right)}=\dfrac{9,5}{402,2}\cdot100\%\approx2,36\%\end{matrix}\right.\)
Theo gt ta có: $n_{MgO}=0,2(mol)$
a, $MgO+2HCl\rightarrow MgCl_2+H_2O$
b, Ta có: $n_{HCl}=0,4(mol)\Rightarrow x=7,3$
Bảo toàn khối lượng ta có: $m_{ddA}=208(g)$
$\Rightarrow \%C_{MgCl_2}=9,13\%$
c, Ta có: $n_{NaOH}=0,2(mol)$
$\Rightarrow n_{Mg(OH)_2}=0,1(mol)$
Bảo toàn khối lượng ta có: $m_{ddB}=208+200-0,1.58=402,2(g)$
$\Rightarrow \%C_{MgCl_2}=2,36\%$

a)
Na + H2O → NaOH + 1/2H2
Dung dịch thu được là dung dịch NaOH
b)
nNa = 4,6 : 23 = 0,2 mol
nH2O = 54 : 18 = 3 mol
=> Na phản ứng hết, nNaOH = nNa = 0,2 mol
<=> mNaOH = 0,2.40 = 8 gam
m dung dịch sau phản ứng = mNa + mH2O - mH2 = 4,6 + 54 - 0,1.2 = 58,4 gam
C% NaOH = \(\dfrac{8}{58,4}.100\)% = 13,7 %

\(n_{Na}=\dfrac{6,9}{23}=0,3\left(mol\right)\\ m_{HCl}=200.3,65\%=7,3\left(g\right)\\ n_{HCl}=\dfrac{7,3}{36,5}=0,2\left(mol\right)\)
\(PTHH:2Na+2HCl\rightarrow2NaCl+H_2\uparrow\\ LTL:0,3>0,2\Rightarrow Na.dư\)
Theo pt: nH2 = 2nHCl = 2.0,2 = 0,4 (mol)
VH2 = 0,4.22,4 = 8,96 (l)
Theo pt: nNaCl = nNa (phản ứng) = nHCl = 0,2 (mol)
=> \(\left\{{}\begin{matrix}m_{NaCl}=0,2.58,5=11,7\left(g\right)\\m_{Na\left(dư\right)}=\left(0,3-0,2\right).23=2,3\left(g\right)\\m_{H_2}=0,4.2=0,8\left(g\right)\end{matrix}\right.\)
=> \(m_{dd}=200+6,9-2,3-0,8=203,8\left(g\right)\)
=> C%NaCl = \(\dfrac{11,7}{203,8}=5,74\%\)

\(n_{Na}=\dfrac{4,6}{23}=0,2\left(mol\right)\\ PTHH:2Na+2H_2O\rightarrow2NaOH+H_2\\ n_{NaOH}=n_{Na}=0,2\left(mol\right);n_{H_2}=\dfrac{0,2}{2}=0,1\left(mol\right)\\ a,V_{H_2\left(đktc\right)}=0,1.22,4=2,24\left(l\right)\\ b,m_{ddNaOH}=4,6+95,6-0,1.2=100\left(g\right)\\ C\%_{ddNaOH}=\dfrac{0,2.40}{100}.100=8\%\)

\(n_{BaSO_4}=\dfrac{23.3}{233}=0.1\left(mol\right)\)
\(Na_2O+H_2O\rightarrow2NaOH\)
\(BaO+H_2O\rightarrow Ba\left(OH\right)_2\)
\(2NaOH+H_2SO_4\rightarrow Na_2SO_4+H_2O\)
\(Ba\left(OH\right)_2+H_2SO_4\rightarrow BaSO_4+H_2O\)
\(n_{BaO}=n_{Ba\left(OH\right)_2}=n_{BaSO_4}=0.1\left(mol\right)\)
\(m_{BaO}=0.1\cdot153=15.3\left(g\right)\)
\(m_{Na_2O}=24.6-15.3=9.3\left(g\right)\)
\(n_{Na_2O}=\dfrac{9.3}{62}=0.15\left(mol\right)\)
\(\%BaO=62.2\%\)
\(\%Na_2O=37.8\%\)
\(2.\)
\(m_{ddX}=24.6+73.7=98.3\left(g\right)\)
\(n_{H_2SO_4}=\dfrac{0.15}{2}+0.1=0.175\left(mol\right)\)
\(m_{dd_{H_2SO_4}}=\dfrac{0.175\cdot98\cdot100}{19.6}=87.5\left(g\right)\)
\(m_{ddY}=m_{ddX}+m_{ddH_2SO_4}-m_{\downarrow}=98.3+87.5-23.3=162.5\left(g\right)\)
\(C\%_{Na_2SO_4}=\dfrac{0.075\cdot142}{162.5}\cdot100\%=6.55\%\)
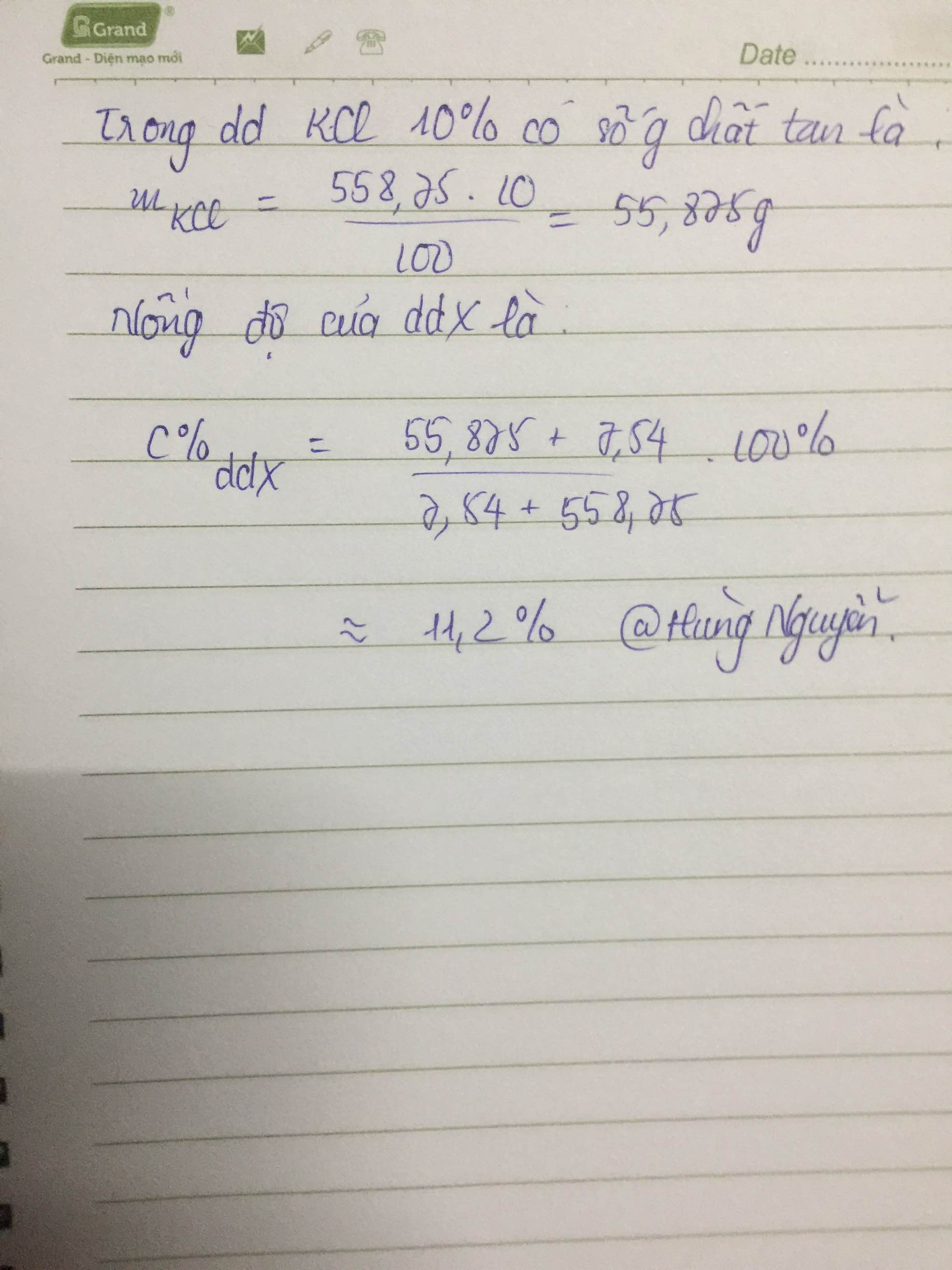
\(n_{MgO}=\dfrac{6}{40}=0,15\left(mol\right)\); \(n_{HNO_3}=\dfrac{100.18,9\%}{63}=0,3\left(mol\right)\)
PTHH: \(MgO+2HNO_3\rightarrow Mg\left(NO_3\right)_2+H_2O\)
Xét tỉ lệ: \(\dfrac{0,15}{1}=\dfrac{0,3}{2}\) => Phản ứng vừa đủ
\(n_{Mg\left(NO_3\right)_2}=0,15\left(mol\right)\)
=> \(m_{Mg\left(NO_3\right)_2}=0,15.148=22,2\left(g\right)\)
\(C\%_{Mg\left(NO_3\right)_2}=\dfrac{22,2}{6+100}.100\%=20,94\%\)