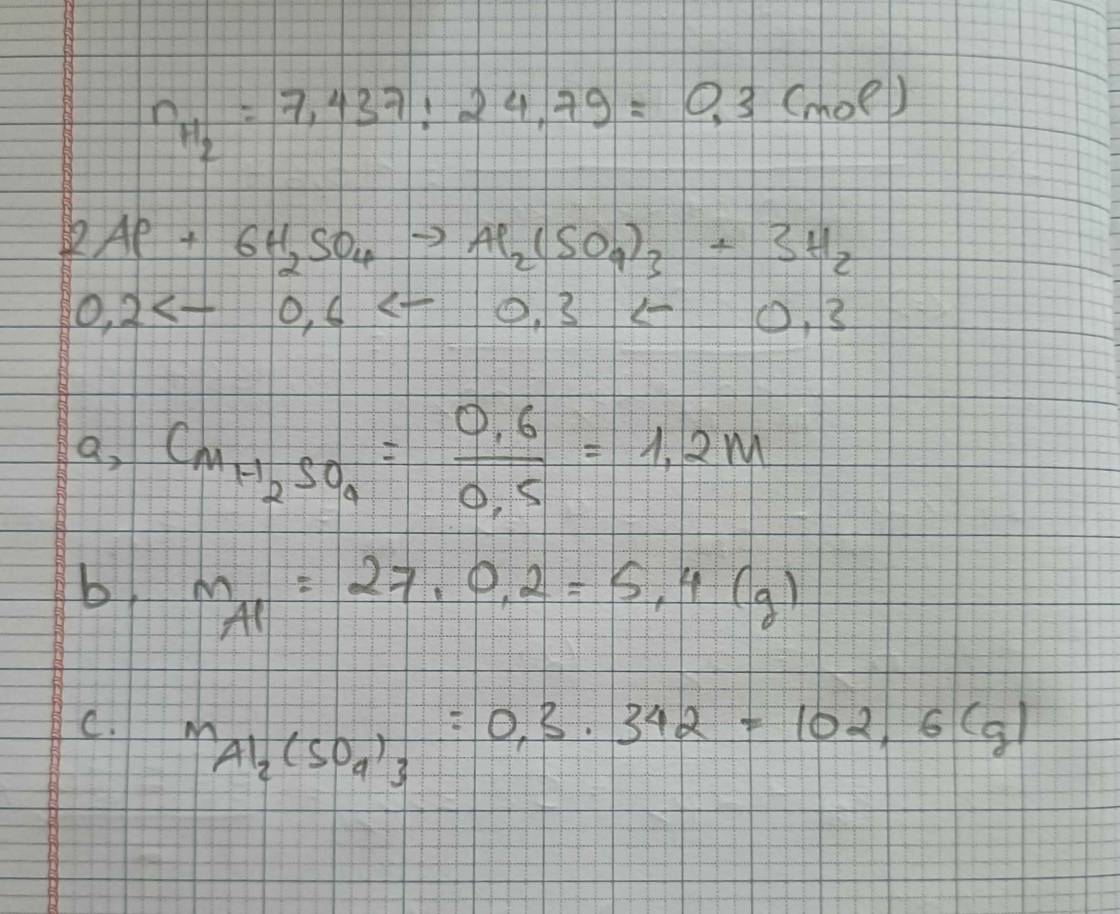Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{Al}=\dfrac{5,4}{27}=0,2\left(mol\right)\\ 2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\\ 0,2........0,3...........0,1...........0,3\left(mol\right)\\ a.C_{MddH_2SO_4}=\dfrac{0,3}{0,3}=1\left(M\right)\\ b.m_{Al_2\left(SO_4\right)_3}=342.0,1=34,2\left(g\right)\\ c.V_{H_2\left(đktc\right)}=0,3.22,4=6,72\left(l\right)\)
PTHH: \(2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\)
a) \(n_{Al}=\dfrac{5,4}{27}=0,2\left(mol\right)\)
\(n_{H_2SO_4}=\dfrac{3}{2}n_{Al}=0,3\left(mol\right)\)
\(\Rightarrow C_{M_{H_2SO_4}}=\dfrac{0,3}{0,3}=1M\)
b) \(n_{Al_2\left(SO_4\right)_3}=\dfrac{1}{2}n_{Al}=0,1\left(mol\right)\)
\(\Rightarrow m_{Al_2\left(SO_4\right)_3}=n.M=0,1.342=34,2\left(g\right)\)
c) \(n_{H_2}=n_{H_2SO_4}=0,3\left(mol\right)\)
\(\Rightarrow V_{H_2}=n.22,4=0,3.22,4=6,72\left(l\right)\)

\(4.a/n_{Al}=\dfrac{5,4}{27}=0,2mol\\ 2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\)
0,2 0,3 0,1 0,3
\(V_{H_2}=0,3.24,79=7,437l\\ b/C_{\%H_2SO_4}=\dfrac{0,3.98}{150}\cdot100=19,6\%\\ c/m_{Al_2\left(SO_4\right)_3}=0,1.342=34,2g\)
\(5.a/n_{MgO}=\dfrac{4}{40}=0,1mol\\ MgO+2HCl\rightarrow MgCl_2+H_2O\)
0,1 0,2 0,1 0,1
\(C_{\%HCl}=\dfrac{0,2.36,5}{200}\cdot100=3,65\%\\ b/C_{\%MgCl_2}=\dfrac{0,1.95}{200+4}\cdot100=4,66\%\\ c/NaOH+HCl\rightarrow NaCl+H_2O\\ n_{NaOH}=n_{HCl}=0,2mol\\ V_{NaOH}=\dfrac{0,2}{1}=0,2l=200ml\)

a) \(n_{Zn}=\frac{m}{M}=\frac{13}{65}=0,2\left(mol\right)\)
Phương trình hóa học phản ứng
Zn + H2SO4 ---> ZnSO4 + H2
1 : 1 : 1 : 1
0.2 0,2 0,2
mol mol mol
=> \(V_{H_2}=n.22,4=0,2.22,4=4,48\left(l\right)\)
b) \(m_{ZnSO_4}=n.M=0,2.161=32,2\left(g\right)\)
c) Ta có \(C\%=\frac{m_{ct}}{m_{dd}}.100\%=24,5\%\)
=> \(m_{ct}=\frac{C\%.m_{dd}}{100\%}=\frac{24,5\%.200}{100\%}=49\left(g\right)=m_{H_2SO_4}\)
=> \(m_{H_2O}=151\left(g\right)\)
=> \(n_{H_2SO_4}=\frac{m}{M}=\frac{49}{98}=0,5\)(mol)
Dễ thấy \(\frac{n_{Zn}}{1}< \frac{n_{H_2SO_4}}{1}\)
=> H2SO4 dư 0,5 - 0,2 = 0,3 (mol)
=> \(m_{H_2SO_4\text{ dư }}=n.M=0,3.98=29,4\left(g\right)\); \(m_{H_2SO4\text{ tham gia}}=n.M=0,2.98=19,6\)(g)
Áp dụng đinhk luật bảo toàn khối lượng
=> \(m_{H_2SO_4}+m_{Zn}=m_{ZnSO4}+m_{H_2}\)
=> \(m_{H_2}=m_{H_2SO_4}+m_{Zn}-m_{ZnSO_4}=19,6+13-32,2=0,4\left(g\right)\)
=> \(m_{saupư}=m_{ZnSO_4}+m_{H_2SO_4\text{ dư}}+m_{H_2O}-m_{H_2}=32,2+29,4+151-0,4=232,2\left(g\right)\)
=> \(C\%_{H_2SO_4}=\frac{m_{ct}}{m_{sau\text{ pư}}}.100\%=\frac{29,4}{232,2}.100\%=12,66\%\)
\(C\%_{ZnSO_4}=\frac{m_{ct}}{m_{dd}}.100\%=\frac{32,2}{232,2}.100\%=13,87\%\)

a) 2Al+6HCl→→2AlCl3+3H2
b)
nAl=10,8\27=0,4(mol)
nAlCl3=nAl=0,4(mol)
mAlCl3=0,4.133,5=53,4(g)
c)
nH2=3\2nAl=0,6(mol)
VH2=22,4.0,6=13,44(l)
d) n HCl=0,4.6\2=1,2 mol
=>Cm HCl=1,2\0,1=12M
\(n_{Al}=\dfrac{10,8}{27}=0,4\left(mol\right)\)
a) Pt : \(2Al+6HCl\rightarrow2AlCl_3+3H_2|\)
2 6 2 3
0,4 1,2 0,4 0,6
b) \(n_{H2}=\dfrac{0,4.3}{2}=0,6\left(mol\right)\)
\(V_{H2\left(dktc\right)}=0,6.24,79=14,874\left(l\right)\)
c) \(n_{AlCl3}=\dfrac{0,6.2}{3}=0,4\left(mol\right)\)
⇒ \(m_{AlCl3}=0,4.133,5=53,4\left(g\right)\)
d) \(n_{HCl}=\dfrac{0,4.6}{2}=1,2\left(mol\right)\)
100ml = 0,1l
\(C_{M_{ddHCl}}=\dfrac{1,2}{0,1}=12\left(M\right)\)
Chúc bạn học tốt

a)
$n_{Al} = 0,3(mol)$
$2Al + 3H_2SO_4 \to Al_2(SO_4)_3 + 3H_2$
Theo PTHH :
$n_{H_2SO_4} = \dfrac{3}{2}n_{Al} = 0,45(mol)$
$m_{dd\ H_2SO_4} = \dfrac{0,45.98}{12,25\%} = 360(gam)$
b)
$n_{H_2} = n_{H_2SO_4} = 0,45(mol)$
$V_{H_2} = 0,45.22,4 = 10,08(lít)$
c)
$n_{Al_2(SO_4)_3} = 0,15(mol)$
$m_{dd\ sau\ pư} = 8,1 + 360 - 0,45.2 = 367,2(gam)$
$C\%_{Al_2(SO_4)_3} = \dfrac{0,15.342}{367,2}.100\% = 14\%$

Mg +H2SO4--->MgSO4 +H2
x x x x mol
Fe+ H2SO4---> FeSO4+ H2
y y y y mol
theo bài ta có : 24x+ 56y=1,36 và x+y=0,672/22,4
=> x=0,01 mol và y=0,02 mol
=> mMg=0,24 gam mFe=1,12 gam
tớ thấy đề bài khó để là ý b) bạn ạ nếu bạn xem lạ đề bài thì tốt quá![]()

\(n_{HCl}=6\cdot0,05=0,3\left(mol\right)\\ a,PTHH:2Al+6HCl\rightarrow2AlCl_3+3H_2\)
Hiện tượng: Al tan dần, có bọt khí không màu xuất hiện
\(b,\left\{{}\begin{matrix}n_{Al}=\dfrac{1}{3}n_{HCl}=0,1\left(mol\right)\\n_{H_2}=\dfrac{1}{2}n_{HCl}=0,15\left(mol\right)\end{matrix}\right.\Rightarrow\left\{{}\begin{matrix}m_{Al}=0,1\cdot27=2,7\left(g\right)\\V_{H_2\left(đkc\right)}=0,15\cdot24,79=3,7185\left(l\right)\end{matrix}\right.\)

a, \(Na_2SO_3+H_2SO_4\rightarrow Na_2SO_4+SO_2+H_2O\)
\(K_2SO_3+H_2SO_4\rightarrow K_2SO_4+SO_2+H_2O\)
Ta có: \(n_{SO_2}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\)
Theo PT: \(n_{H_2SO_4}=n_{SO_2}=0,3\left(mol\right)\)
\(\Rightarrow m_{ddH_2SO_4}=\dfrac{0,3.98}{20\%}=147\left(g\right)\)
b, Ta có: 126nNa2SO3 + 158nK2SO3 = 44,2 (1)
Theo PT: \(n_{SO_2}=n_{Na_2SO_3}+n_{K_2SO_3}=0,3\left(2\right)\)
Từ (1) và (2) \(\Rightarrow\left\{{}\begin{matrix}n_{Na_2SO_3}=0,1\left(mol\right)\\n_{K_2SO_3}=0,2\left(mol\right)\end{matrix}\right.\)
Có: m dd sau pư = 44,2 + 147 - 0,3.64 = 172 (g)
\(\Rightarrow\left\{{}\begin{matrix}C\%_{Na_2SO_3}=\dfrac{0,1.126}{172}.100\%\approx7,33\%\\C\%_{K_2SO_3}=\dfrac{0,2.158}{172}.100\%\approx18,37\%\end{matrix}\right.\)
c, \(n_{Ba\left(OH\right)_2}=0,5.1=0,5\left(mol\right)\)
\(\Rightarrow\dfrac{n_{SO_2}}{n_{Ba\left(OH\right)_2}}=0,6< 1\) → Pư tạo BaSO3.
PT: \(SO_2+Ba\left(OH\right)_2\rightarrow BaSO_3+H_2O\)
\(n_{BaSO_3}=n_{SO_2}=0,3\left(mol\right)\Rightarrow m_{BaSO_3}=0,3.217=65,1\left(g\right)\)