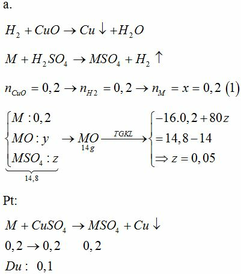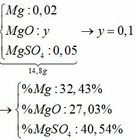Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

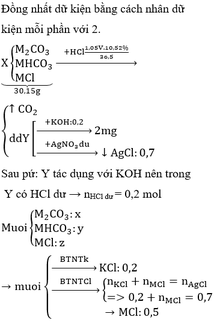
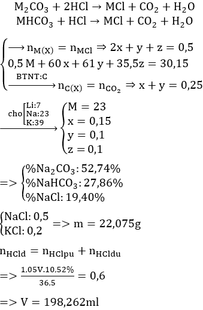
43,71 gam hỗn hợp: \(\left\{{}\begin{matrix}M_2CO_3:a\left(mol\right)\\MHCO_3:b\left(mol\right)\\MCl:c\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow60\left(a+b\right)+M\left(2a+b+c\right)+b+35,5c=43,71\left(I\right)\)
\(M_2CO_3\left(a\right)+2HCl\left(2a\right)\rightarrow2MCl\left(2a\right)+CO_2\left(a\right)+H_2O\)
\(MHCO_3\left(b\right)+HCl\left(b\right)\rightarrow MCl\left(b\right)+CO_2\left(b\right)+H_2O\)
Dung dịch A: \(\left\{{}\begin{matrix}MCl:2a+b+c\left(mol\right)\\HCl\left(dư\right)\end{matrix}\right.\)
Khí B là \(CO_2:\left(a+b\right)mol\)
\(n_{CO_2}=0,4\left(mol\right)\)
\(\Rightarrow a+b=0,4\left(II\right)\)
Gọi d là số mol HCl dư
- Phần 1:
\(MCl\left(a+0,5b+0,5c\right)+AgNO_3\rightarrow AgCl\left(a+0,5b+0,5c\right)+MNO_3\)
\(HCl\left(0,5d\right)+AgNO_3\rightarrow AgCl\left(0,5d\right)+HNO_3\)
\(n_{AgCl}=0,48\left(mol\right)\)
\(\Rightarrow a+0,5b+0,5c+0,5d=0,48\left(III\right)\)
- Phần 2:
Cho phần 2 qua dd KOH thì chỉ có HCl dư tdung
\(HCl\left(0,5d\right)+KOH\left(0,5d\right)\rightarrow KCl\left(0,5d\right)+H_2O\)
\(\Rightarrow0,5d=0,1\)
\(\Rightarrow d=0,2\)
Thay vào (III) => \(a+0,5b+0,5c=0,38\left(IV\right)\)
29,68 gam Muối khan: \(\left\{{}\begin{matrix}MCl:a+0,5b+0,5c\left(mol\right)\\KCl:0,5d=0,1\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow M\left(a+0,5b+0,5c\right)+35,5\left(a+0,5b+0,5c\right)+7,45=29,68\)
Thay (IV) vào \(\Leftrightarrow0,38M=8,74\)
\(\Leftrightarrow M=23\left(Na\right)\)
\(\Rightarrow\left\{{}\begin{matrix}b+35,5c=2,23\\a+b=0,4\\a+0,5b+0,5c=0,38\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}a=0,3\\b=0,1\\c=0,06\end{matrix}\right.\)
=> %m mỗi muối
\(\sum n_{HCl}=n_{HCl}\left(pư\right)+n_{HCl}\left(dư\right)=2a+b+d=0,9\left(mol\right)\)
\(\Rightarrow\)V dung dịch HCl.

Gọi số mol của Fe và Cu trong hỗn hợp lần lượt là x và y.
2Fe + 3Cl2 → 2FeCl3 (1)
x(mol) x(mol)
Cu + Cl2 → CuCl2 (2)
y(mol) y(mol)
Fe + 2HCl → FeCl2 + H2 (3)
x (mol) 2x(mol) x(mol).
Theo điều kiện bài toán và phương trình hoá học (3) ta có: 127x = 25,4 → x = 0,2
Theo phương trình phản ứng (1) và (2) ta có: 162,5x + 135y = 59,5
Vậy y = 0,2.
Khối lượng mỗi muối là: m FeCl3=32,5gam
m CuCl2=27gam
%FeCl3 = 54,62%.
%CuCl2 = 45,38%

3. CuO +H2SO4 -->CuSO4 +H2O
nCuO=64/80=0,8(mol)
theo PTHH :nCuO =nH2SO4=nCuSO4=0,8(mol)
=>mddH2SO4 20%=0,8.98.100/20=392(g)
mCuSO4=0,8.160=128(g)
mdd sau phản ứng =64 +392=456(g)
mH2O=456 -128=328(g)
giả sử có a g CuSO4.5H2O tách ra
trong 250g CuSO4 tách ra có 160g CuSO4 và 90g H2O tách ra
=> trong a g CuSO4.5H2O tách ra có : 160a/250 g CuSO4 và 90a/250 g H2O tách ra
=>mCuSO4(còn lại)=128 -160a/250 (g)
mH2O (còn lại)=328 -90a/250 (g)
=>\(\dfrac{128-\dfrac{160a}{250}}{328-\dfrac{90a}{250}}.100=25\)
=>a=83,63(g)

MgCO3 ----> MgO + CO2
CaCO3 -----> CaO + CO2
0,15 (mol) <------------ 0,15 (mol) (1) đây ý nói là tổng lượng mol CO2 = tổng lượng hỗn hợp muối
MgCO3 + HCl -------> MgCl2 + CO2 + H20
CaCO3 + HCl --------> CaCl2 + CO2 + H20
=> n(MgCO3,CaCO3) = n(MgCl2,CaCl2) = 0,15 (mol)
=> M(MgCl2,CaCl2) = 317/3
Sau đó, ta đặt: C (là phần trăm của CaCl2 trong hỗn hợp muối)
1-C (là phần trăm của MgCl2 trong hỗn hợp muối)
Với C là 100% trong hỗn hợp đó
=> 111C + 95x(1-C) = 317/3
Từ đó suy ra: C= 2/3
Vì lượng muối trong hỗn hợp tác dụng với HCl bằng lượng từng muối trong hỗn hợp ban đầu nên
%CaCO3 = 2/3x100% = 66,667%
%MgCO3 = 1/3x100% = 33,33%