Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a. PTHH: S + O2 ---to---. SO2 (1)
Ta có: \(n_S=\dfrac{9,6}{32}=0,3\left(mol\right)\)
Theo PT(1): \(n_{SO_2}=n_S=0,3\left(mol\right)\)
=> \(V_{SO_2}=0,3.22,4=6,72\left(lít\right)\)
b. PTHH: SO2 + Ca(OH)2 ---> CaSO3↓ + H2O (2)
Theo PT(2): \(n_{CaSO_3}=n_{SO_2}=0,3\left(mol\right)\)
=> \(m_{CaSO_3}=0,3.120=36\left(g\right)\)
\(n_S=\dfrac{9,6}{32}=0,3\left(mol\right)\)
PTHH: S + O2 ---to→ SO2
Mol: 0,3 0,3
\(V_{SO_2}=0,3.22,4=6,72\left(l\right)\)
PTHH: SO2 + Ca(OH)2 → CaSO3 + H2O
Mol: 0,3 0,3
\(m_{CaSO_3}=0,3.120=36\left(g\right)\)

\(n_{SO2}=\dfrac{6,4}{64}=0,1\left(mol\right)\)
Pt : \(S+O_2\rightarrow\left(t_o\right)SO_2|\)
1 1 1
0,1 0,1
\(n_{O2}=\dfrac{0,1.1}{1}=0,1\left(mol\right)\)
\(V_{O2\left(lt\right)}=0,1.22,4=2,24\left(l\right)\)
⇒ \(V_{O2\left(tt\right)}=\dfrac{2,24.100}{80}=2,8\left(l\right)\)
Chúc bạn học tốt

Hình như để sai thiệt đó bạn. Minh tinh mai no no cu ra am. neu ra duong thi lai ra n=0

Gọi x,y lần lượt là số mol Mg, Fe
Mg + S ⟶ MgS
Fe + S ⟶ FeS
MgS + 4H2SO4 → MgSO4 + 4H2O + 4SO2
2FeS + 10H2SO4 → Fe2(SO4)3 + 9SO2 + 10H2O
S + 2H2SO4 → 3SO2 + 2H2O
Ta có :
\(\left\{{}\begin{matrix}Mg:x\left(mol\right)\\Fe:y\left(mol\right)\end{matrix}\right.\underrightarrow{+S:0,5\left(mol\right)}\left\{{}\begin{matrix}MgS:x\left(mol\right)\\FeS:y\left(mol\right)\\S_{dư}:0,5-\left(x+y\right)\left(mol\right)\end{matrix}\right.\underrightarrow{+H_2SO_4}\left\{{}\begin{matrix}MgSO_4:x\left(mol\right)\\Fe_2\left(SO_4\right)_3:\dfrac{y}{2}\left(mol\right)\\SO_2\end{matrix}\right.\underrightarrow{+NaOH\left(dư\right)}\left(kt\right)\left\{{}\begin{matrix}Mg\left(OH\right)_2:x\left(mol\right)\\Fe\left(OH\right)_3:y\left(mol\right)\end{matrix}\right.\underrightarrow{to}\left\{{}\begin{matrix}MgO:x\left(mol\right)\\Fe_2O_3:\dfrac{y}{2}\left(mol\right)\end{matrix}\right.\)
Ta có :\(n_{SO_2}=4x+4,5y+\left[0,5-\left(x+y\right)\right].3=2\left(mol\right)\)
\(40x+160\dfrac{y}{2}=24\)
=> \(\left\{{}\begin{matrix}x=0,2\\y=0,2\end{matrix}\right.\)
=> \(m_{Mg}=0,2.24=4,8\left(g\right)\)
\(m_{Fe}=0,2.56=11,2\left(g\right)\)
\(m=4,8+11,2=16\left(g\right)\)
\(\%m_{Mg}=\dfrac{4,8}{16}.100=30\%\)
\(\%m_{Fe}=100-30=70\%\)

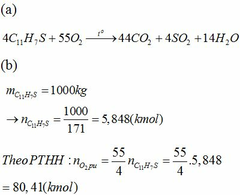
Do lấy dư 20% oxi so với lượng cần đốt cháy nên lượng oxi đã lấy là:


Tổng khối lượng CO2 và SO2 :
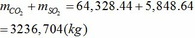
Chú ý:
Lượng O2 lấy dư 20% so với với lượng cần thiết => tính mol O2 chính xác
\(n_{SO_2}=\dfrac{2,24}{22,4}=0,1\left(mol\right)\)
PTHH: S + O2 --to--> SO2
0,1<--------------0,1
=> \(\%S=\dfrac{32.0,1}{3,4}.100\%=94,12\%\)
=> B