Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a, \(Fe+H_2SO_4\rightarrow FeSO_4+H_2\)
\(n_{H_2}=\dfrac{7,437}{24,79}=0,3\left(mol\right)\)
Theo PT: \(n_{Fe}=n_{H_2}=0,3\left(mol\right)\Rightarrow m_{Fe}=0,3.56=16,8\left(g\right)\)
b, \(n_{H_2SO_4}=n_{H_2}=0,3\left(mol\right)\Rightarrow C\%_{H_2SO_4}=\dfrac{0,3.98}{120}.100\%=24,5\%\)
c, m dd sau pư = 16,8 + 120 - 0,3.2 = 136,2 (g)
d, \(n_{FeSO_4}=n_{H_2}=0,3\left(mol\right)\)
\(\Rightarrow C\%_{FeSO_4}=\dfrac{0,3.152}{136,2}.100\%\approx33,48\%\)

a) \(n_{Cl_2}=\dfrac{4,48}{22,4}=0,2\left(mol\right)\)
PTHH: 2Fe + 3Cl2 --to--> 2FeCl3
_____\(\dfrac{2}{15}\)<--0,2------------->\(\dfrac{2}{15}\)
=> mFe = \(\dfrac{2}{15}.56=7,467\left(g\right)\)
b) \(m_{FeCl_3}=\dfrac{2}{15}.162,5=21,667\left(g\right)\)

Phương trình hoá học : 2Fe + 3 Cl 2 → t ° FeCl 3
Theo định luật bảo toàn khối lượng :
m Fe + m Cl 2 = m FeCl 3
m Cl 2 = m FeCl 3 - m Fe = 16,25 - 5,6 = 10,65g

a, PT: \(CH_4+2O_2\underrightarrow{t^o}CO_2+2H_2O\)
\(C_2H_4+3O_2\underrightarrow{t^o}2CO_2+2H_2O\)
Ta có: \(n_{CH_4}+n_{C_2H_4}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\left(1\right)\)
Theo PT: \(n_{CO_2}=n_{CH_4}+2n_{C_2H_4}=\dfrac{8,96}{22,4}=0,4\left(mol\right)\left(2\right)\)
Từ (1) và (2) \(\Rightarrow\left\{{}\begin{matrix}n_{CH_4}=0,2\left(mol\right)\\n_{C_2H_4}=0,1\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}\%V_{CH_4}=\dfrac{0,2.22,4}{6,72}.100\%\approx66,67\%\\\%V_{C_2H_4}\approx33,33\%\end{matrix}\right.\)
b, Theo PT: \(n_{O_2}=2n_{CH_4}+3n_{C_2H_4}=0,7\left(mol\right)\)
\(\Rightarrow V_{O_2}=0,7.22,4=15,68\left(l\right)\)

500mlh2so4=0.5l ; nh2=33,6/22,4=1,5 mol
ptpu: 2Fe+3H2SO4 --->Fe2(SO4)3 +3H2
2mol 3mol 1 mol 3mol
1mol 1mol 1.5 mol
b/ mfe =1.56=56g
c/ cm=n/v =>n=cm.v=1.98=98

a. \(n_{CH_4}=\dfrac{4.48}{22,4}=0,2\left(mol\right)\)
PTHH : CH4 + 2O2 ---t0---> CO2 + 2H2O
0,2 0,4 0,2
b. \(V_{O_2}=0,4.22,4=8,96\left(l\right)\)
\(V_{CO_2}=0,2.22,4=4,48\left(l\right)\)
c. \(V_{kk}=8,96.5=44,8\left(l\right)\)

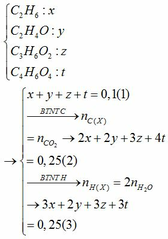
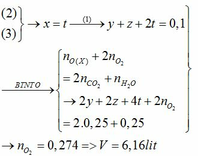
$2Fe + 3Cl_2 \xrightarrow{t^o} 2FeCl_3$
$n_{FeCl_3} = \dfrac{32,5}{162,5} = 0,2(mol)$
$n_{Cl_2} = \dfrac{3}{2}n_{FeCl_3} = 0,3(mol)$
$V_{Cl_2} = 0,3.22,4 = 6,72(lít)$