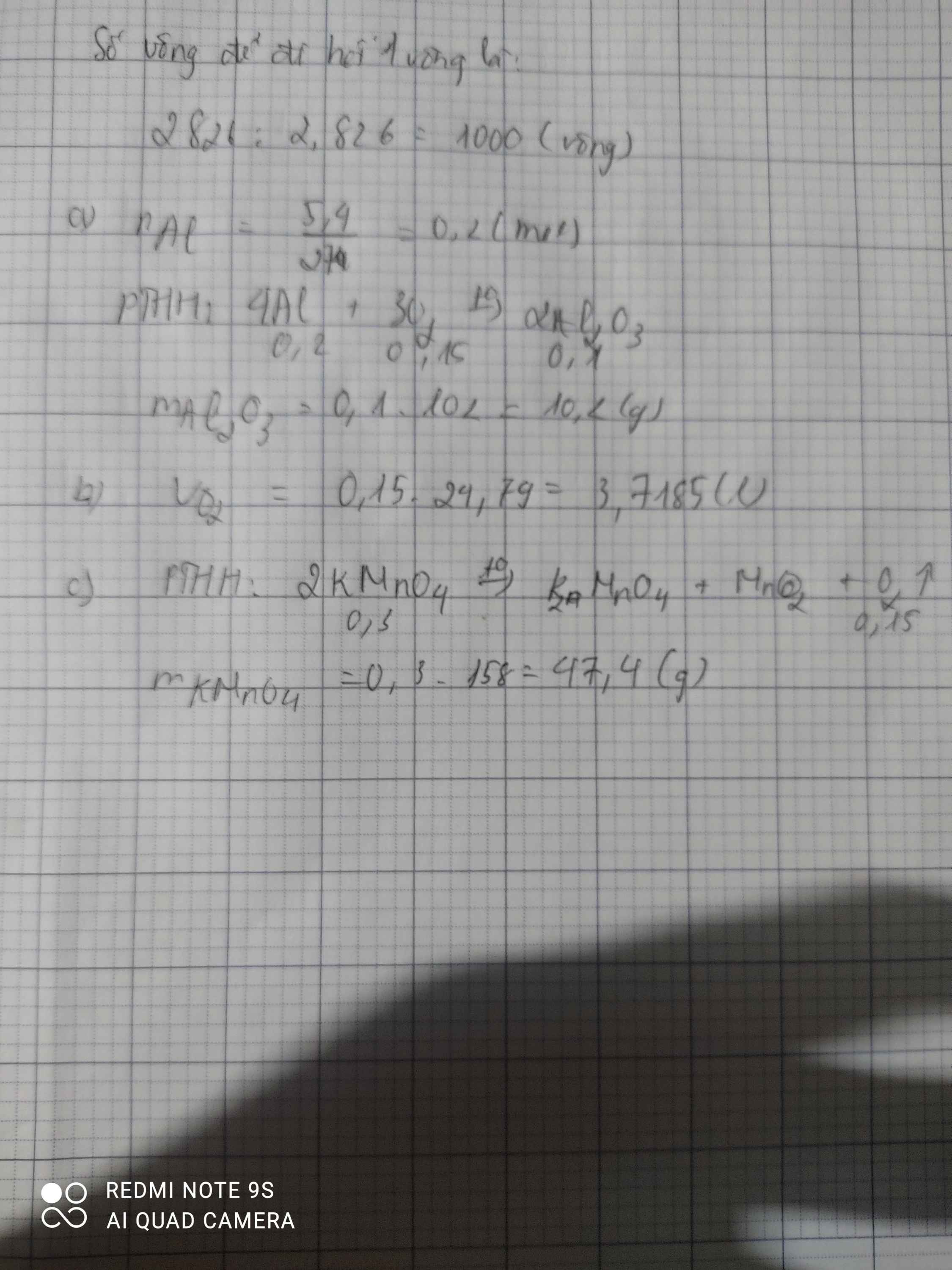Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(a,PTHH:4Al+3O_2\rightarrow\left(t^o\right)2Al_2O_3\\ b,n_{Al_2O_3}=\dfrac{30,6}{102}=0,3\left(mol\right)\\ n_{Al}=\dfrac{4}{2}.n_{Al_2O_3}=2.0,3=0,6\left(mol\right)\\ \Rightarrow m_{Al}=0,6.27=16,2\left(g\right)\\ c,n_{O_2}=\dfrac{3}{2}.n_{Al_2O_3}=\dfrac{3}{2}.0,3=0,45\left(mol\right)\\ \Rightarrow V_{O_2\left(đkc\right)}=0,45.24,79=11,1555\left(l\right)\)
a, \(4Al+3O_2\underrightarrow{t^o}2Al_2O_3\)
b, \(nAl_2O_3=\dfrac{30,6}{102}=0,3\left(mol\right)\)
\(nAl=\dfrac{4}{2}.0,3=0,6\left(mol\right)\)
\(mAl=0,6.27=16,2\left(g\right)\)
c, \(nO_2=\dfrac{3}{2}.0,3=0,45\left(mol\right)\)
\(VO_{2\left(đkc\right)}=0,45.24,79=11,1555\left(l\right)\)

\(n_{Al}=\dfrac{0,54}{27}=0,02\left(mol\right)\)
Pt : \(4Al+3O_2\xrightarrow[]{t^o}2Al_2O_3\)
0,02-->0,015-->0,01
a) \(m_{Al2O3}=0,01.102=1,02\left(g\right)\)
b) \(V_{O2\left(dktc\right)}=0,015.24,79=0,37185\left(l\right)\)
sửa lại \(V_{\left(dktc\right)}-->V_{\left(dkc\right)}\)

1. \(n_{O_2}=\dfrac{2,479}{24,79}=0,1\left(mol\right)\)
\(4Al+3O_2\underrightarrow{t^o}2Al_2O_3\)
Theo PT: \(n_{Al}=\dfrac{4}{3}n_{O_2}=\dfrac{2}{15}\left(mol\right)\Rightarrow m_{Al}=\dfrac{2}{15}.27=3,6\left(g\right)\)
2. \(n_{KCl\left(25\%\right)}=300.25\%=75\left(g\right)\)
Gọi: m dd KCl 10% = a (g) ⇒ mKCl (10%) = 10%a (g)
\(\Rightarrow\dfrac{75+10\%a}{a+300}=0,15\Rightarrow a=600\left(g\right)\)

a. Aluminium + Khí oxygen -> Aluminium oxide
b. \(m_{Al}+m_O=m_{Al_{2_{ }}O_3}\)
c. Từ câu b => \(m_{Al}=m_{Al_{2_{ }}O_3}-m_O=20.4-9.6=10.8\)
Phương trình chữ:
aluminium + oxygen \(\rightarrow\) aluminium oxide
Biểu thức khối lượng:
\(m_{Al}+m_{O_2}=m_{Al_2O_3}\)
Khối lượng aluminium:
\(m_{Al}=m_{Al_2O_3}-m_{O_2}=20,4-9,6=10,8g\)

a, \(4Al+3O_2\underrightarrow{t^o}2Al_2O_3\)
b, Gọi: mO2 = x (g) ⇒ mAl = 1,5x (g)
Theo ĐLBT KL, có: mAl + mO2 = mAl2O3
⇒ 1,5x + x = 10
⇒ x = 4 (g) = mO2
mAl = 1,5.4 = 6 (g)

1. Theo ĐLBT KL, có: mAl + mO2 = mAl2O3
2. \(n_{Al_2O_3}=\dfrac{10,2}{102}=0,1\left(mol\right)\)
PT: \(4Al+3O_2\underrightarrow{t^o}2Al_2O_3\)
Theo PT: \(n_{O_2}=\dfrac{3}{2}n_{Al_2O_3}=0,15\left(mol\right)\)
\(\Rightarrow m_{O_2}=0,15.32=4,8\left(g\right)\)

a. \(n_{KMnO_4}=\dfrac{47.4}{158}=0,3\left(mol\right)\)
PTHH : 2KMnO4 ---to----> K2MnO4 + MnO2 + O2
0,3 0,15
\(V_{O_2}=0,15.22,4=3,36\left(l\right)\)
b. PTHH : 4Al + 3O2 -> 2Al2O3
0,2 0,15
\(m_{Al}=0,2.27=5,4\left(g\right)\)

a) \(n_{Al}=\dfrac{5,4}{27}=0,2\left(mol\right)\)
PTHH: 4Al + 3O2 --to--> 2Al2O3
0,2-->0,15
=> V = 0,15.22,4 = 3,36 (l)
b)
PTHH: 2KMnO4 --to--> K2MnO4 + MnO2 + O2
0,3<----------------------------0,15
=> mKMnO4(lý thuyết) = 0,3.158 = 47,4 (g)
=> \(m_{KMnO_4\left(tt\right)}=\dfrac{47,4.110}{100}=52,14\left(g\right)\)