Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

n Hcl pu la 0,95*2 = 0,39 mol
n Hcl du la 0,5 -0,39 = 0,11 mol
gọi v lít là thể tích dung dịch kiềm
n Naoh la 0,2V mol,nBaoh la 0,1V mol
pthh .....bạn ghi ra 2 pthh giua naoh voi hcl,baoh vs hcl
Ta co 0,4V =0,11
suy ra V =0,275 L

Câu 7 :
\(n_{H2SO4}=0,1.1=0,1\left(mol\right)\)
Pt : \(H_2SO_4+2NaOH\rightarrow Na_2SO_4+2H_2O\)
\(n_{NaOH}=2n_{H2SO4}=2.0,1=0,2\left(mol\right)\Rightarrow V_{ddNaOH}=\dfrac{0,2}{1}=0,2\left(l\right)=200\left(ml\right)\)
Câu 8 :
\(n_{H2SO4}=0,5.0,7=0,35\left(mol\right)\)
Pt : \(H_2SO_4+2KOH\rightarrow K_2SO_4+H_2O\)
\(n_{KOH}=2n_{H2SO4}=2.0,35=0,7\left(mol\right)\)
\(\Rightarrow m_{ddKOH}=\dfrac{0,7.56}{12\%}.100\%=326,67\left(g\right)\)
\(\Rightarrow V_{ddKOH}=\dfrac{326,67}{1,15}=284,06\left(ml\right)\)
Câu 12 :
a) \(2Cu+O_2\xrightarrow[]{t^o}2CuO\)
\(CuO+2HCl\rightarrow CuCl_2+H_2O\)
\(CuCl_2+2NaOH\rightarrow Cu\left(OH\right)_2+2NaCl\)
\(Cu\left(OH\right)_2\xrightarrow[]{t^o}CuO+H_2O\)
\(CuO+H_2SO_4\rightarrow CuSO_4+H_2O\)
\(CuSO_4+Fe\rightarrow FeSO_4+Cu\downarrow\)
b) \(MgSO_4+2KOH\rightarrow Mg\left(OH\right)_2+K_2SO_4\)
\(Mg\left(OH\right)_2\xrightarrow[]{t^o}MgO+H_2O\)
\(MgO+2HCl\rightarrow MgCl_2+H_2O\)
\(MgCl_2+2AgNO_3\rightarrow Mg\left(NO_3\right)_2+2AgCl\)
\(Mg\left(NO_3\right)_2+Na_2CO_3\rightarrow MgCO_3+2NaNO_3\)
\(MgCO_3\xrightarrow[]{t^o}MgO+CO_2\)
c) \(2Na+2H_2O\rightarrow2NaOH+H_2\)
\(NaOH=HCl\rightarrow NaCl+H_2O\)
\(2NaCl+2H_2O\xrightarrow[cmn]{đpdd}2NaOH+H_2+Cl_2\)
\(Cl_2+H_2\xrightarrow[]{as}2HCl\)
\(HCl+Fe\rightarrow FeCl_2+H_2\)
\(FeCl_2+2NaOH\rightarrow Fe\left(OH\right)_2+2NaCl\)
\(Fe\left(OH\right)_2+H_2SO_4\rightarrow FeSO_4+2H_2O\)
\(FeSO_4+BaCl_2\rightarrow FeCl_2+BaSO_4\)
\(FeCl_2+2AgNO_3\rightarrow Fe\left(NO_3\right)_2+2AgCl\)
\(Fe\left(NO_3\right)_2+Mg\rightarrow Mg\left(NO_3\right)_2+Fe\)
e) \(4Al+3O_2\xrightarrow[]{t^o}2Al_2O_3\)
\(Al_2O_3+6HCl\rightarrow2AlCl_3+3H_2O\)
\(Al_2\left(SO_4\right)_3+6KOH\rightarrow2Al\left(OH\right)_3+3K_2SO_4\)
\(Al\left(OH\right)_3+3HNO_3\rightarrow Al\left(NO_3\right)_3+3H_2O\)
\(Al\left(NO_3\right)_2+Mg\rightarrow Mg\left(NO_3\right)_2+Âl\)
\(2Al+3Cl_2\xrightarrow[]{t^o}2AlCl_3\)
Bạn xem đề chỗ AlCl3 ra Al2(SO4)3 nhé

\(n_{CO_2} = 0,1(mol)\)
CaCO3.MgCO3 + 4HCl → CaCl2 + MgCl2 + 2CO2 + 2H2O(1)
........0,05...............0,2.......................0,05.......0,1..........................(mol)
\(n_{NaOH}= 0,12(mol)\)
MgCl2 + 2NaOH → Mg(OH)2 + 2NaCl(2)
..0,1...........0,2.................................................(mol)
HCl + NaOH → NaCl + H2O(3)
0,02.....0,02................................(mol)
Theo PTHH (1)(3) suy ra :
\(n_{HCl} = 0,2 + 0,02 = 0,22(mol)\\ \Rightarrow V_{dd\ HCl} = \dfrac{0,22}{1} = 0,22(lít)\)
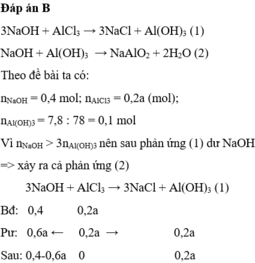

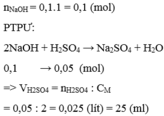
Ta có theo đề bài: nMgCl2=0,2\(\times\)1=0,2(mol)
theo đề bài ta có pthh: MgCl2 +2NaOH\(\rightarrow\)Mg(OH)2+2NaCl
theo pthh trên thì nMgCl2\(\times\)2=nNaCl=0,2\(\times\)2=0,4(mol)
V dd NaOH=0,4\(\div\)2=0,2(l)=200(ml)
vậy cần 200ml ddNaOH 2M thì hòa tan hết 200 ml dd MgCl2 1M
PTHH: MgCl2+2NaOH--->Mg(OH)2+2NaCl
nMgCl2= 1.0,2= 0,2 mol
Theo pt: nNaOH=2.nMgCl2=2.0,2= 0,4 mol
=> Vdd NaOH= \(\dfrac{0,4}{2}\)= 0,2 l