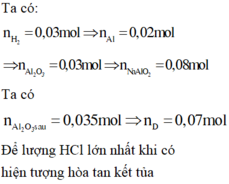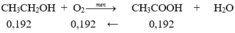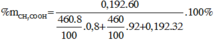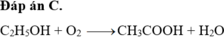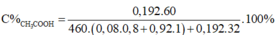Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{SO_4^{2-}}=2.n_{BaSO_4\downarrow}=0,3\left(mol\right)\Rightarrow\left[SO_4^{2-}\right]=\dfrac{0,3}{0,4}=0,75M\)
\(n_{NH_4^+}=2.n_{NH_3\uparrow}=\dfrac{2.0,22}{22,4}\approx0,02\left(mol\right)\Rightarrow\left[NH_4^+\right]=\dfrac{0,02}{0,4}=0,05M\)
\(n_{Mg}=2.n_{Mg\left(OH\right)_2\downarrow}=\dfrac{2.46,55}{58}\approx1,6\left(mol\right)\Rightarrow\left[Mg^{2+}\right]=\dfrac{1,6}{0,4}=4M\)
Bảo toàn điện tích:
\(n_{NO_3^-}=2.1,6+0,05-0,3.2=2,65\left(mol\right)\Rightarrow\left[NO_3^-\right]=\dfrac{2,65}{0,4}=6,625M\)
Đề lỗi không vậy?

m C2H5OH = m.29,87% = 0,2987m(gam)
=> n C2H5OH = 0,2987m/46 (mol)
m H2O = m - 0,2987m = 0,7013m(gam)
=> n H2O = 0,7013m/18(mol)
$2C_2H_5OH + 2Na \to 2C_2H_5ONa + H_2$
$2Na + 2H_2O \to 2NaOH + H_2$
n H2 = 1/2 n C2H5OH + 1/2 H2O = 11,76/22,4 = 0,525(mol)
=> 1/2 . 0,2987m/46 + 1/2 . 0,7013m/18 = 0,525
=> m = 23,1(gam)
Suy ra :
m C2H5OH = 0,2987.23,1 = 6,9(gam)
V C2H5OH = 6,9/0,8 = 8,625(ml)
m H2O = 0,7013.23,1 = 16,2(gam)
V H2O = 16,2/1 = 16,2(ml)
Vậy :
Đr = V C2H5OH / V(dd) .100 = 8,625/(8,625 + 16,2) .100 = 34,74o

n A l : n A l 2 O 3 = 2 : 3 n A l = 2 n H 2 3 = 0 , 02 ⇒ n A l 2 o 3 = 0 , 03
Sơ đồ: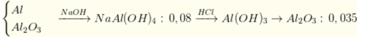
⇒ n H C l = 0 , 07 + ( 0 , 08 - 0 , 07 ) . 4 = 0 , 11 ⇒ [ H C l ] = 0 , 11 0 , 2 = 0 , 55
Đáp án C

\(n_{H_2}\approx0,35\left(mol\right)\)
\(n_{Cl_2}=0,375\left(mol\right)\)
\(Fe+2HCl-->FeCl_2+H_2\uparrow\)
x.........2x........................x..............x
\(2M+2nHCl-->2MCl_n+nH_2\uparrow\)
4x..........4xn.................4x................2xn
\(2M+nCl_2-->2MCl_n\)
4x.......2xn...................4x
\(2Fe+3Cl_2-->2FeCl_3\)
x..........1,5x..............x
\(x+2xn=\dfrac{7,84}{22,4}\Rightarrow2xn=\dfrac{7,84}{22,4}-x\left(1\right)\)
\(2xn+1,5x=0,375\left(2\right)\)
thay(1) vaog(2) => x=0,05
n=3
Thể tích Cl2 tác dụng vs M
\(V_{Cl_2}=2.3.0,05.22,4=6,72\left(l\right)\)
b) \(M=\dfrac{5,4}{4.0,05}=27\left(\dfrac{g}{mol}\right)\)
=> M: Al
\(n_{H_2}=0,1\left(mol\right)\)
\(V_{HCl}=400\left(ml\right)=0,4\left(l\right)\)
\(Zn+2HCl-->ZnCl_2+H_2\uparrow\)
0,1.......0,2...................0,1.............0,1
\(CM_{HCl}=\dfrac{0,2}{0,4}=0,5\left(M\right)\)
\(m_{ZnCl_2}=0,1.136=13,6\left(g\right)\)
b) \(n_{KOH}=\dfrac{50.22,4\%}{56.100\%}=0,2\left(mol\right)\)
\(n_{HCl}=0,2.0,5=0,1\left(mol\right)\)
\(HCl+KOH-->KCl+H_2O\)
\(\dfrac{0,1}{1}< \dfrac{0,2}{1}\) =>KOH dư
\(V_{KOH}=\dfrac{50}{1,25}=40\left(ml\right)=0,04\left(l\right)\)
\(CM_{KCl}=\dfrac{0,1}{0,2+0,04}=\dfrac{5}{12}\left(M\right)\)
\(CM_{KOH}=\dfrac{0,2-0,1}{0,2+0,04}=\dfrac{5}{12}\left(M\right)\)