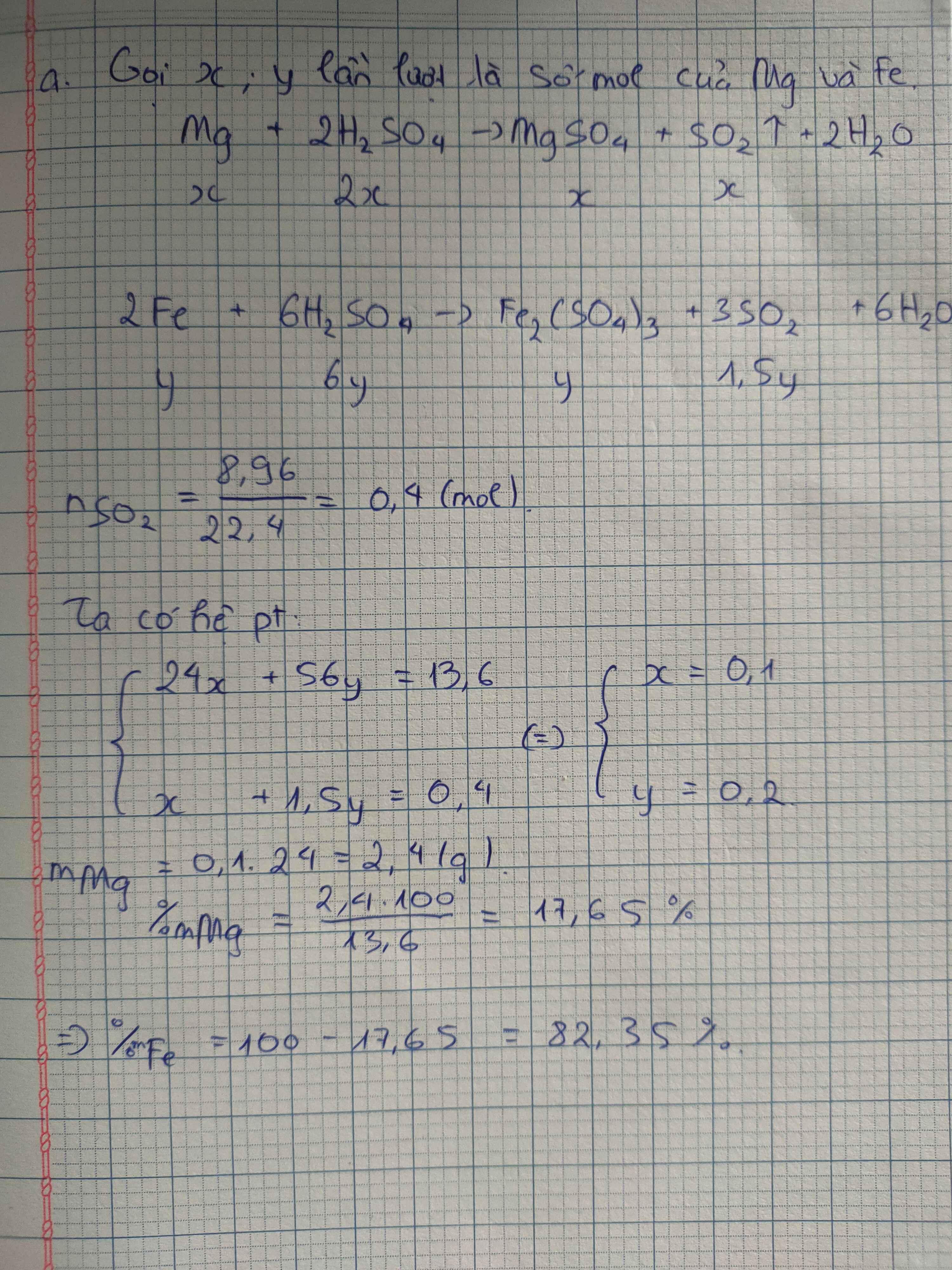Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a/ \(n_{SO_2}=\dfrac{3,08}{22,4}=0,1375\left(mol\right);n_{H_2}=\dfrac{1,68}{22,4}=0,075\left(mol\right)\)
2Fe + 6H2SO4(đ) ---to---> Fe2(SO4)3 + 6SO2 + 3H2O
x 3x
Cu + 2H2SO4(đ) ---to---> CuSO4 + SO2 + 2H2O
y y
Fe + 2HCl ----> FeCl2 + H2
x x
Cu + 2HCl -----> CuCl2 + H2
y y
Ta có hệ pt: \(\left\{{}\begin{matrix}3x+y=0,1375\\x+y=0,075\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}x=0,03125\left(mol\right)\\y=0,04375\left(mol\right)\end{matrix}\right.\)
\(m_{hh}=0,03125.56+0,04375.64=4,55\left(g\right)\)
\(\%m_{Fe}=\dfrac{0,03125.56.100\%}{4,55}=38,46\%\)
b, \(n_{Ba\left(OH\right)_2}=0,1.1,2=0,12\left(mol\right)\)
Ta có: \(T=\dfrac{n_{SO_2}}{n_{Ba\left(OH\right)_2}}=\dfrac{0,1375}{0,12}=1,1458\)
=> tạo ra 2 muối là BaSO3 và Ba(HSO3)2
SO2 + Ba(OH)2 ---> BaSO3 + H2O
x x x
2SO2 + Ba(OH)2 ----> Ba(HSO3)2
y 0,5y 0,5y
Ta có hệ pt: \(\left\{{}\begin{matrix}x+y=0,1375\\x+0,5y=0,12\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}x=0,1025\left(mol\right)\\y=0,035\left(mol\right)\end{matrix}\right.\)
\(m_{muối}=0,1025.217+0,5.0,035.299=27,475\left(g\right)\)

PTHH: \(2Fe+6H_2SO_{4\left(đ\right)}\underrightarrow{t^o}Fe_2\left(SO_4\right)_3+3SO_2\uparrow+6H_2O\)
\(CuO+H_2SO_4\rightarrow CuSO_4+H_2O\)
a) Ta có: \(n_{SO_2}=\dfrac{2,24}{22,4}=0,1\left(mol\right)\) \(\Rightarrow n_{Fe}=\dfrac{1}{15}\left(mol\right)\)
\(\Rightarrow\%m_{Fe}=\dfrac{\dfrac{1}{15}\cdot56}{13,6}\cdot100\%\approx27,45\%\) \(\Rightarrow\%m_{CuO}=72,55\%\)
b) Ta có: \(m_{CuO}=13,6-\dfrac{1}{15}\cdot56\approx9,9\left(g\right)\) \(\Rightarrow n_{CuO}=n_{H_2SO_4}=\dfrac{9,9}{80}=0,12375\left(mol\right)\)
*Làm gì có H2SO4 loãng đâu nhỉ ??

\(n_{Fe}=a\left(mol\right),n_{FeO}=b\left(mol\right)\)
\(m_X=56a+72b=12.8\left(g\right)\)
\(n_{H_2}=n_{Fe}=\dfrac{2.24}{22.4}=0.1\left(mol\right)\)
\(\Rightarrow a=0.1\)
\(b=\dfrac{12.8-56\cdot0.1}{72}=0.1\left(mol\right)\)
\(BTe:\)
\(3n_{Fe}+n_{FeO}=2n_{SO_2}\)
\(\Rightarrow n_{SO_2}=\dfrac{3\cdot0.1+0.1}{2}=0.2\left(mol\right)\)
\(V_{SO_2}=0.2\cdot22.4=4.48\left(l\right)\)
\(\)

\(n_{SO_2}=\dfrac{2.8}{22.4}=0.125\left(mol\right)\)
\(n_{Fe}=a\left(mol\right),n_{Zn}=b\left(mol\right)\)
\(m=56a+65b=6.05\left(g\right)\left(1\right)\)
\(\text{Bảo toàn e : }\)
\(3a+2b=0.125\cdot2=0.25\left(2\right)\)
\(\left(1\right),\left(2\right):\)
\(a=b=0.05\)
\(\%Fe=\dfrac{0.05\cdot56}{6.05}\cdot100\%=46.28\%\)
\(\%Zn=53.72\%\)

Coi : B gồm : Fe ( x mol) , O ( y mol)
\(m_B=56x+16y=12\left(h\right)\left(1\right)\)
\(n_{SO_2}=\dfrac{3.36}{22.4}=0.15\left(mol\right)\)
Bảo toàn e :
\(3x=2y+0.15\cdot2\left(2\right)\)
\(\left(1\right),\left(2\right):x=0.18,y=0.12\)
\(m_{Fe}=0.18\cdot56=10.08\left(g\right)\)
Quy đổi hỗn hợp về Fe và O.
Giả sử: \(\left\{{}\begin{matrix}n_{Fe}=x\left(mol\right)\\n_O=y\left(mol\right)\end{matrix}\right.\)
⇒ 56x + 16y = 12 (1)
Ta có: \(n_{SO_2}=0,15\left(mol\right)\)
Theo ĐLBT mol e, có: 3x - 2y = 0,15.2 (2)
Từ (1) và (2) \(\Rightarrow\left\{{}\begin{matrix}x=0,18\left(mol\right)\\y=0,12\left(mol\right)\end{matrix}\right.\)
⇒ mFe = 0,18.56 = 10,08 (g)
Bạn tham khảo nhé!