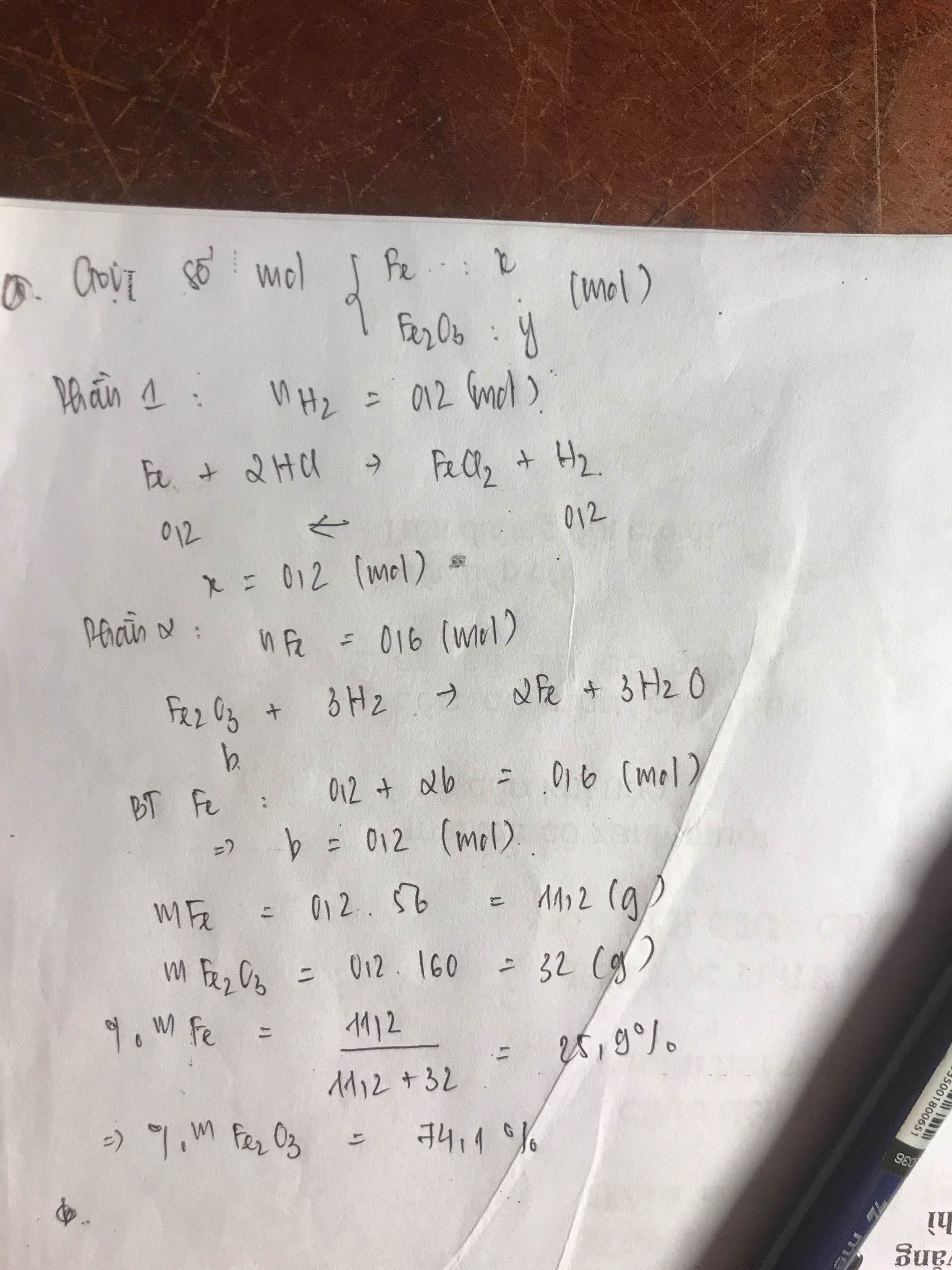Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

Bài 4:
a)
Gọi số mol CO, H2 trong mỗi phần là a, b (mol)
=> 28a + 2b = 1,14 (1)
+ Phần 1:
PTHH: Fe2O3 + 3H2 --to--> 2Fe + 3H2O
\(\dfrac{1}{3}b\)<----b-------->\(\dfrac{2}{3}b\)
Fe2O3 + 3CO --to--> 2Fe + 3CO2
\(\dfrac{1}{3}a\)<---a----------->\(\dfrac{2}{3}a\)
=> \(\dfrac{2}{3}a+\dfrac{2}{3}b=\dfrac{6,72}{56}=0,12\)
=> a + b = 0,18 (2)
(1)(2) => a = 0,03 (mol); b = 0,15 (mol)
=> \(\left\{{}\begin{matrix}\%V_{CO}=\dfrac{0,03}{0,03+0,15}.100\%=16,67\%\\\%V_{H_2}=\dfrac{0,15}{0,03+0,15}.100\%=83,33\%\end{matrix}\right.\)
b) \(n_{Fe_2O_3}=\dfrac{1}{3}a+\dfrac{1}{3}b=0,06\left(mol\right)\)
=> mFe2O3 = 0,06.160 = 9,6 (g)
c)
\(n_{O_2}=\dfrac{11,2.20\%}{22,4}=0,1\left(mol\right)\)
\(n_{N_2}=\dfrac{11,2.80\%}{22,4}=0,4\left(mol\right)\)
PTHH: 2CO + O2 --to--> 2CO2
0,03->0,015-->0,03
2H2 + O2 --to--> 2H2O
0,15->0,075
=> B chứa \(\left\{{}\begin{matrix}CO_2:0,03\left(mol\right)\\O_2:0,1-\left(0,015+0,075\right)=0,01\left(mol\right)\\N_2:0,4\left(mol\right)\end{matrix}\right.\)
=> \(\overline{M}_B=\dfrac{0,03.44+0,01.32+0,4.28}{0,03+0,01+0,4}=\dfrac{321}{11}\left(g/mol\right)\)
=> \(d_{B/C_2H_6}=\dfrac{\dfrac{321}{11}}{30}=\dfrac{107}{110}\)

Giả sử các khí được đo ở điều kiện sao cho 1 mol khí chiếm thể tích 1 lít
Gọi số mol CH4, C2H6 là a, b (mol)
=> \(a+b=\dfrac{25}{1}=25\left(mol\right)\) (1)
\(n_{O_2}=\dfrac{95}{1}=95\left(mol\right)\)
PTHH: CH4 + 2O2 --to--> CO2 + 2H2O
a---->2a---------->a
2C2H6 + 7O2 --to--> 4CO2 + 6H2O
b------>3,5b-------->2b
=> \(\left\{{}\begin{matrix}n_{O_2\left(dư\right)}=95-2a-3,5b\left(mol\right)\\n_{CO_2}=a+2b\left(mol\right)\end{matrix}\right.\)
=> \(95-a-1,5b=\dfrac{60}{1}=60\)
=> a + 1,5b = 35 (2)
(1)(2) => a = 5; b = 20
=> \(\left\{{}\begin{matrix}\%V_{CH_4}=\dfrac{5}{25}.100\%=20\%\\\%V_{C_2H_6}=\dfrac{20}{25}.100\%=80\%\end{matrix}\right.\)
\(\overline{M}_A=\dfrac{5.16+20.30}{5+20}=27,2\left(g/mol\right)\)
\(\overline{M}_B=20,5.2=41\left(g/mol\right)\)
=> \(d_{A/B}=\dfrac{27,2}{41}\approx0,663\)

a) \(n_{CH_4}=\dfrac{1,12}{22,4}=0,05\left(mol\right)\)
\(n_{CO_2}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\)
PTHH: CH4 + 2O2 --to--> CO2 + 2H2O
0,05-->0,1------->0,05
2C2H2 + 5O2 --to--> 4CO2 + 2H2O
0,125<--0,3125<----0,25
=> \(\left\{{}\begin{matrix}\%V_{CH_4}=\dfrac{0,05}{0,05+0,125}.100\%=28,57\%\\\%V_{C_2H_2}=\dfrac{0,125}{0,05+0,125}.100\%=71,43\%\end{matrix}\right.\)
\(\left\{{}\begin{matrix}\%m_{CH_4}=\dfrac{0,05.16}{0,05.16+0,125.26}.100\%=19,753\%\\\%m_{C_2H_2}=\dfrac{0,125.26}{0,05.16+0,125.26}.100\%=80,247\%\end{matrix}\right.\)
b) \(n_{O_2}=0,1+0,3125=0,4125\left(mol\right)\)
=> \(V_{O_2}=0,4125.22,4=9,24\left(l\right)\)
=> Vkk = 9,24.5 = 46,2 (l)

\(Đặt:\)
\(n_{CO\left(p1\right)}=a\left(mol\right),n_{_{ }H_2\left(p1\right)}=b\left(mol\right)\)
\(n_{CO\left(p2\right)}=ka\left(mol\right),n_{_{ }H_2\left(p1\right)}=kb\left(mol\right)\)
\(m_{mp}=284a\left(k+1\right)+2b\left(k+1\right)=18.3\left(g\right)\)
\(\Rightarrow\left(k+1\right)\left(28a+2b\right)=18.3\left(1\right)\)
\(P1:\)
\(2CO+O_2\underrightarrow{t^0}2CO_2\)
\(2H_2+O_2\underrightarrow{t^0}2H_2O\)
\(n_{O_2}=\dfrac{a}{2}+\dfrac{b}{2}=0.25\left(mol\right)\)
\(\Rightarrow a+b=0.5\left(2\right)\)
\(P2:\)
\(BTKL:\)
\(52+ka\cdot28+kb\cdot2=38.4+ka\cdot44+kb\cdot18\)
\(\Rightarrow16ka+16kb=13.6\)
\(\Rightarrow16k\left(a+b\right)=13.6\)
\(\Rightarrow k=\dfrac{13.6}{16\cdot0.5}=1.7\)
\(Từ\left(1\right):\)
\(\Rightarrow28a+2b=\dfrac{18.3}{2.7}=\dfrac{61}{9}\left(3\right)\)
\(\)\(\left(2\right),\left(3\right):\)
\(a=\dfrac{2}{9},b=\dfrac{5}{18}\)
\(\%CO=\dfrac{\dfrac{2}{9}\cdot2.7}{\dfrac{2}{9}\cdot2.7+\dfrac{5}{18}\cdot2.7}\cdot100\%=44.44\%\cdot\)
\(\%H_2=55.56\%\)
Giải ra số lẻ , không biết mình làm đúng hay sai nhưng bạn xem thử nhé !!
Phần 1 :
\(2CO + O_2 \xrightarrow{t^o} 2CO_2\\ 2H_2 + O_2 \xrightarrow{t^o} 2H_2O\\ n_{CO} + n_{H_2} = 2n_{O_2} = 2.\dfrac{5,6}{22,4} = 0,25.2 = 0,5\)
Ta có :
\(n_{O(oxit)\ pư} = \dfrac{52-38,4}{16} = 0,85(mol)\\ CO + O_{oxit} \to CO_2 \\ H_2 + O_{oxit} \to H_2O\\ n_{CO} + n_{H_2} = n_O = 0,85(mol)\)
Vậy :
\(n_A = n_{CO} + n_{H_2} = 0,5 + 0,85 = 1,35(mol)\\ m_A = 28n_{CO} + 2n_{H_2} = 18,3(gam)\\ \Rightarrow n_{CO} = 0,6 ; n_{H_2} = 0,75\\ \Rightarrow \%V_{CO} = \dfrac{0,6}{1,35}.100\% = 44,44\%\\ \%V_{H_2} = 100\%-44,44\% = 55,56\%\)

a)
$n_{Cl_2} : n_{O_2} = 1 : 2$
Suy ra :
$\%V_{Cl_2} = \dfrac{1}{1 + 2}.100\% = 33,33\%$
$\%V_{O_2} = \dfrac{2}{1 + 2}.100\% = 66,67\%$
b)
Coi $n_{Cl_2} = 1 (mol) \Rightarrow n_{O_2} = 2(mol)$
$\%m_{Cl_2} = \dfrac{1.71}{1.71 + 2.32}.100\% = 52,59\%$
$\%m_{O_2} = 100\% -52,59\% = 47,41\%$
c)
$M_A = \dfrac{71.1 + 32.2}{1 + 2} = 45(g/mol)$
$d_{A/B} = \dfrac{45}{28} = 1,607$