Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

PTHH: \(3Fe+2O_2\underrightarrow{t^o}Fe_3O_4\)
Ta có: \(\left\{{}\begin{matrix}n_{Fe}=\dfrac{11,2}{56}=0,2\left(mol\right)\\n_{O_2}=\dfrac{12,8}{32}=0,4\left(mol\right)\end{matrix}\right.\)
Xét tỉ lệ: \(\dfrac{0,2}{3}< \dfrac{0,4}{2}\) \(\Rightarrow\) Oxi còn dư, Fe p/ứ hết
\(\Rightarrow n_{O_2\left(dư\right)}=0,4-\dfrac{2}{15}=\dfrac{4}{15}\left(mol\right)\)
+) Theo PTHH: \(\left\{{}\begin{matrix}n_{O_2}=\dfrac{2}{15}\left(mol\right)\\n_{Fe_3O_4}=\dfrac{1}{15}\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}V_{kk}=\dfrac{2}{15}\cdot22,4\cdot5\approx14,93\left(l\right)\\m_{Fe_3O_4}=\dfrac{1}{15}\cdot232\approx15,47\left(g\right)\end{matrix}\right.\)

\(a,n_{H_2}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\\ n_{O_2}=\dfrac{8,96}{22,4}=0,4\left(mol\right)\)
PTHH: \(2H_2+O_2\underrightarrow{t^o}2H_2O\)
bđ 0,3 0,4
pư 0,3 0,15
sau pư 0 0,25 0,3
=> H2 hết, O2 dư
\(m_{O_2\left(dư\right)}=0,25.32=8\left(g\right)\)
b) \(A_{H_2O}=0,3.6.10^{23}=1,8.10^{23}\left(phân.tử\right)\)
c) \(m_{O_2\left(pư\right)}=0,15.32=4,8\left(g\right)\)
PTHH: \(2KMnO_4\underrightarrow{t^o}K_2MnO_4+MnO_2+O_2\)
0,3<-------------------------------------0,15
\(\rightarrow m_{KMnO_4}=0,3.158=47,4\left(g\right)\)

a) \(n_{Na}=\dfrac{9,2}{23}=0,4\left(mol\right);n_{O_2}=\dfrac{1,12}{22,4}=0,05\left(mol\right)\)
PTHH: 4Na + O2 --to--> 2Na2O
Xét tỉ lệ: \(\dfrac{0,4}{4}>\dfrac{0,05}{1}\) => Na dư, O2 hết
PTHH: 4Na + O2 --to--> 2Na2O
0,2<-0,05------>0,1
=> \(m_{Na\left(dư\right)}=\left(0,4-0,2\right).23=4,6\left(g\right)\)
b) \(\left\{{}\begin{matrix}\%m_{Na\left(dư\right)}=\dfrac{4,6}{9,2+0,05.32}.100\%=42,6\%\\\%m_{Na_2O}=\dfrac{0,1.62}{9,2+0,05.32}.100\%=57,4\%\end{matrix}\right.\)

a: \(n_{Na}=\dfrac{9.2}{23}=0.4\left(mol\right)\)
\(n_{O_2}=\dfrac{1.12}{22.4}=0.05\left(mol\right)\)
=>Na dư 0,35 mol
b: \(4Na+O_2\rightarrow2Na_2O\)

nP=\(\dfrac{62}{31}\)=0,2(mol)
nO2=\(\dfrac{7,84}{22,4}\)=0,35(mol)
PTHH:4P+5O2to→2P2O5
tpứ: 0,2 0,35
pứ: 0,2 0,25 0,1
spứ: 0 0,1 0,1
a)chất còn dư là oxi
mO2dư=0,1.32=3,2(g)
b)mP2O5=n.M=0,1.142=14,2(g)
\(a.n_P=0,2\left(mol\right);n_{O_2}=0,35\left(mol\right)\\ 4P+5O_2-^{t^o}\rightarrow2P_2O_5\\ LTL:\dfrac{0,2}{4}< \dfrac{0,35}{5}\\ \Rightarrow SauphảnứngO_2dư\\ n_{O_2\left(pứ\right)}=\dfrac{5}{4}n_P=0,25\left(mol\right)\\ \Rightarrow m_{P\left(dư\right)}=\left(0,35-0,25\right).32=3,2\left(g\right)\\ b.n_{P_2O_5}=\dfrac{1}{2}n_P=0,1\left(mol\right)\\ \Rightarrow m_{P_2O_5}=0,1.142=14,2\left(g\right)\)

a. \(n_P=\frac{6,2}{31}=0,2mol\)
\(V_{O_2}=V_{kk}.\frac{1}{5}=\frac{18,48}{5}=3,696l\)
\(n_{O_2}=\frac{3,696}{22,4}=0,165mol\)
PTHH: \(4P+5O_2\xrightarrow{t^o}2P_2O_5\)
Tỷ lệ \(\frac{0,2}{4}>\frac{0,165}{5}\)
Vậy P dư
\(n_{P\left(\text{phản ứng }\right)}=\frac{4}{5}n_{O_2}=0,132mol\)
\(n_{P\left(dư\right)}=0,2-0,132=0,068mol\)
\(\rightarrow m_{P\left(dư\right)}=0,068.31=2,108g\)
b. \(n_{P_2O_5}=\frac{2}{5}n_{O_2}=0,066mol\)
\(\rightarrow m_{P_2O_5}=0,066.142=9,372g\)
c. PTHH: \(2KClO_3\xrightarrow{t^o}2KCl+3O_2\)
\(n_{KClO_3}=\frac{2}{3}n_{O_2}=0,11mol\)
\(\rightarrow m_{KClO_3}=0,11.122,5=13,475g\)
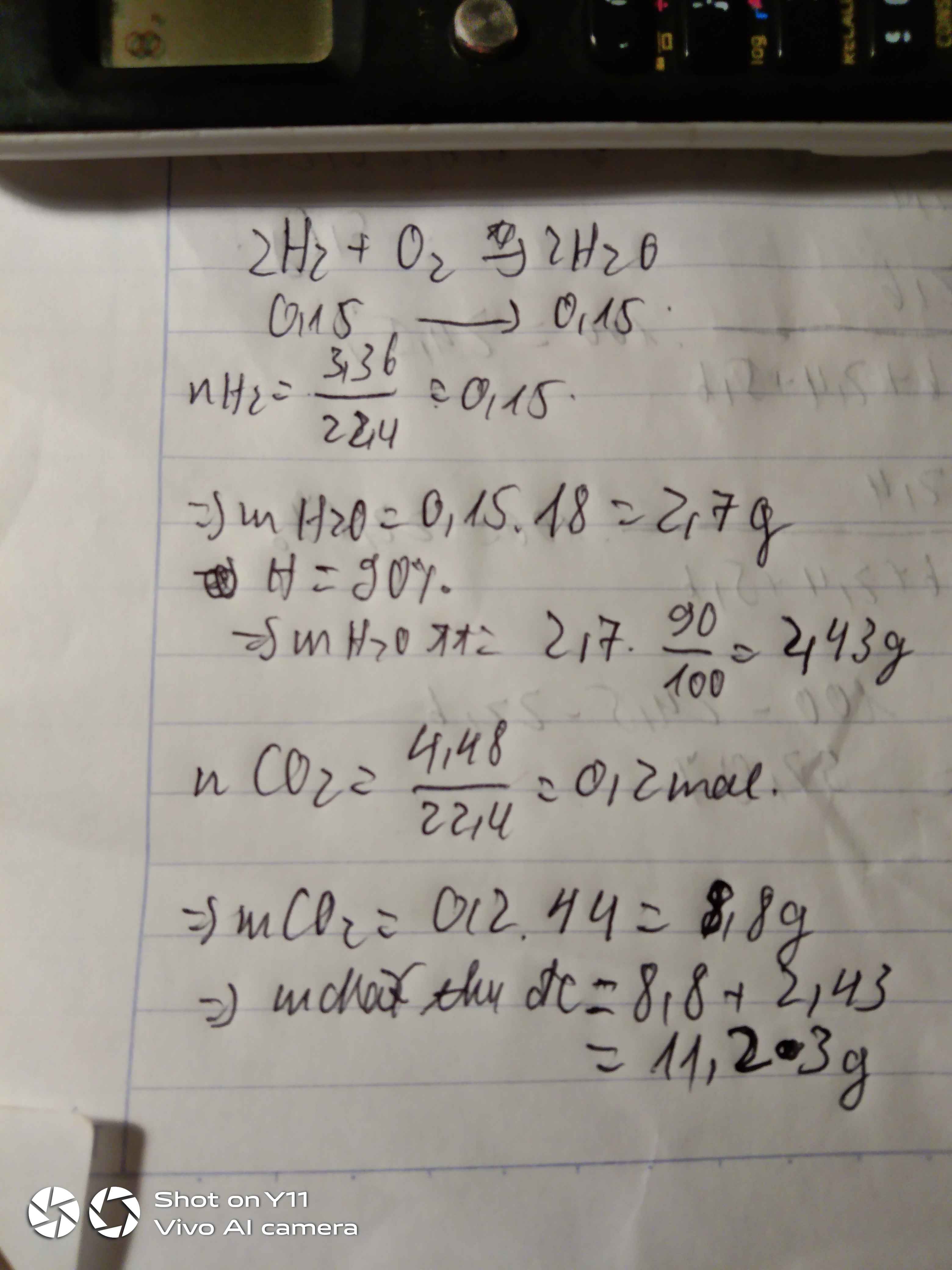
1_)
Theo đề bài ta có : \(nH2=\dfrac{5,6}{22,4}=0,25\left(mol\right)\)
PTHH:
\(2H2+O2-^{t0}->2H2O\)
0,25mol......................0,25mol
=> mH2O = 0,25.18 = 4,5(g)
2_)
Theo đề ta có : nH2 = \(\dfrac{13,44}{22,4}=0,6\left(mol\right)\)
PTHH :
\(2H2+O2-^{t0}->2H2O\)
0,6mol...0,3mol
=> VO2(đktc) = 0,3.22,4 = 6,72(l)
3_)
Theo đề ta có : \(nH2=nO2=\dfrac{11,2}{22,4}=0,5\left(mol\right)\)
PTHH:
\(2H2+O2-^{t0}->2H2O\)
0,5mol...0,25mol
Theo PTHH ta có : nH2 = \(\dfrac{0,5}{2}mol< nO2=\dfrac{0,5}{1}mol=>nO2\left(d\text{ư}\right)\) ( tính theo nH2)
=> mO2(dư) = (0,5-0,25).32= (g)
1, 2H2O---> 2H2+O2
nH2= \(\dfrac{5,6}{22,4}\)= 0,25 mol
Theo pt: nH2O= nH2= 0,25 mol
=> mH2O= 0,25.18= 4,5 g