Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

PTHH: \(C_2H_5OH+3O_2\xrightarrow[]{t^o}2CO_2+3H_2O_{ }\)
\(CO_2+Ca\left(OH\right)_2\rightarrow CaCO_3\downarrow+H_2O\)
a) Ta có: \(n_{CaCO_3}=\dfrac{160}{100}=1,6\left(mol\right)=n_{CO_2}\) \(\Rightarrow n_{O_2}=2,4\left(mol\right)\)
\(\Rightarrow V_{kk}=\dfrac{2,4\cdot22,4}{20\%}=268,8\left(l\right)\)
b) Theo PTHH: \(n_{C_2H_5OH}=\dfrac{1}{2}n_{CO_2}=0,8\left(mol\right)\)
\(\Rightarrow a=C\%_{C_2H_5OH}=\dfrac{0,8\cdot46}{50\cdot0,8}\cdot100\%=92\%=92^o\)

\(n_{C_2H_5OH}=\dfrac{9,2}{46}=0,2\left(mol\right)\)
\(C_2H_5OH+3O_2\underrightarrow{t^o}2CO_2+2H_2O\)
0,2 0,6 0,4 0,4
\(a,V_{O_2}=0,6.22,4=13,44\left(l\right)\)
\(V_{kk}=13,44.5=67,2\left(l\right)\)
b, \(CO_2+Ca\left(OH\right)_2\rightarrow CaCO_3\downarrow+H_2O\)
0,4 0,4
\(m_{CaCO_3}=0,4.100=40\left(g\right)\)
\(m_{CaCO_3tt}=40.95\%=38\left(g\right)\)

\(a,n_{C_6H_{12}O_6}=\dfrac{36}{180}=0,2\left(mol\right)\)
PTHH: \(C_6H_{12}O_6\underrightarrow{\text{men rượu}}2C_2H_5OH+2CO_2\uparrow\)
0,2----------------->0,4----------->0,4
=> VCO2 = 0,4.22,4 = 8,96 (l)
b, mC2H5OH = 0,4.46.50% = 9,2 (g)
\(c,V_{C_2H_5OH}=\dfrac{9,2}{0,8}=11,5\left(ml\right)\\ \rightarrow V_{ddC_2H_5OH}=\dfrac{11,5.100}{60}=\dfrac{115}{6}\left(ml\right)\)

a, \(C_2H_6O+3O_2\underrightarrow{t^o}2CO_2+3H_2O\)
b, \(n_{C_2H_6O}=\dfrac{23}{46}=0,5\left(mol\right)\)
Theo PT: \(n_{O_2}=3n_{C_2H_6O}=1,5\left(mol\right)\)
\(\Rightarrow V_{O_2}=1,5.22,4=33,6\left(l\right)\)
c, \(V_{C_2H_6O}=\dfrac{100.46}{100}=46\left(ml\right)\)
\(\Rightarrow m_{C_2H_6O}=46.0,8=36,8\left(g\right)\)
\(\Rightarrow n_{C_2H_6O}=\dfrac{36,8}{46}=0,8\left(mol\right)\)
PT: \(C_2H_5OH+Na\rightarrow C_2H_5ONa+\dfrac{1}{2}H_2\)
Theo PT: \(n_{H_2}=\dfrac{1}{2}n_{C_2H_5ONa}=0,4\left(mol\right)\)
\(\Rightarrow V_{H_2}=0,4.22,4=8,96\left(l\right)\)

\(n_{CaCO_3}=\dfrac{100,2}{100}=1,002\left(mol\right)\)
PTHH: Ca(OH)2 + CO2 ---> CaCO3 + H2O
1,002 <---- 1,002
C2H5OH + 3O2 --to--> 2CO2 + 3H2O
0,501 <-------------------- 1,002
\(\rightarrow m_{C_2H_5OH}=0,501.46=23,046\left(g\right)\\ \rightarrow V_{C_2H_5OH}=\dfrac{23,046}{0,8}=28,8075\left(ml\right)\)
=> Độ rượu là: \(\dfrac{29,8075}{30}=96,025^o\)

\(n_{CO2}=\dfrac{4,48}{22,4}=0,2\left(mol\right)\)
\(C_2H_5OH+3O_2\xrightarrow[]{t^o}2CO_2+3H_2O\)
0,1 0,3 0,2
b) \(m_{C2H5OH}=0,1.46=4,6\left(g\right)\)
c) \(V_{O2\left(dktc\right)}=0,3.22,4=6,72\left(l\right)\)
Chúc bạn học tốt

\(V_{C_2H_5OH}=\dfrac{20.96}{100}=19,2\left(ml\right)\)
=> \(m_{C_2H_5OH}\) = 19,2.0,8 = 15,36 (g)
=> \(n_{C_2H_5OH}=\dfrac{15,36}{46}=\dfrac{192}{575}\left(mol\right)\)
PTHH: \(C_2H_5OH+3O_2\underrightarrow{t^o}2CO_2+3H_2O\)
Theo PTHH: \(n_{O_2}=\dfrac{576}{575}\left(mol\right)\Rightarrow V_{O_2\left(đktc\right)}=\dfrac{576}{575}.22,4=\dfrac{12902,4}{575}\left(l\right)\)
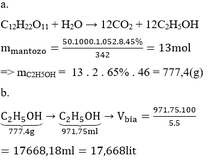
1. Theo đề ta có : nCaCO3 = nCO2 = 1,6(mol)
PTHH :
\(C2H6O+3O2-^{t0}->2CO2+3H2O\)
0,8mol........2,4mol..............1,6mol
CO2 + ca(OH)2 - > CaCO3 + H2O
a) Vkk = 5VO2 = 5.2,4.22,4 = 268,8(l)
b) Ta có : m(rượu) = 0,8.46 = 36,8(g) => V(rượu) = \(\dfrac{36,8}{0,8}=46\left(ml\right)\)
=> độ rượu = \(\dfrac{V\left(rượu-ng-chất\right)}{Vdd\left(rượu\right)}.100=\dfrac{46}{50}.100=92^0\)
PTHH :
\(C6H12O6\xrightarrow[30-33^{0C}]{men-rượu}\) 2C2H5OH + 2CO2
0,375mol..........................0,75mol......0,75mol
a) mC6H12O6(cần dùng) = 0,375.180 =67,5(g)
b) Ta có :
V(rượu ng chất ) = \(\dfrac{0,75.46}{0,8}=43,125\left(ml\right)\)
=> V(rượu 46 độ ) = \(\dfrac{43,125.100}{46}=93,75\left(ml\right)\)