 Giúp mình các phương trình này với
Giúp mình các phương trình này với
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


\(a.CH\equiv C-CH_3+AgNO_3+NH_3->AgC\equiv C-CH_3+NH_4NO_3\\ b.nCH_3-C\left(CH_3\right)=CH-CH_3\underrightarrow{t^{^{ }0},xt,p}\left(-C\left(CH_3\right)_2-CH\left(CH_3\right)-\right)_n\\ c.CH_3-C\left(CH_3\right)=CH-CH_3+HOH\underrightarrow{t^{^{ }0}}CH_3-COH\left(CH_3\right)-CH_2-CH_3\\ d.HC\equiv CH+HOH\underrightarrow{xt,t^{^{ }0}}CH_3CHO\\ e.CH_2=CH-CH_3+HBr->\left[CH_3-CHBr-CH_3;CH_2Br-CH_2-CH_3\right]\\ f.CH_2=CH-CH_2-CH_3+Br_2->CH_2Br-CHBr-CH_2-CH_3\\ g.C_3H_8+Cl_2\underrightarrow{as,1:1}C_3H_7Cl+HCl\\ h.C_4H_{10}\underrightarrow{t^{^0},xt,p}C_4H_8+H_2\\ i.CH_3-CH=CH-CH_3+Br_2->CH_3-CHBr-CHBr=CH_3\)

a, \(C_2H_6+Cl_2\underrightarrow{as}C_2H_5Cl+HCl\)
b, \(nCH_2=CH-CH_3\underrightarrow{t^o}\left(-CH_2-CH\left(CH_3\right)-\right)_n\)
c, \(CH_2=CH-CH_3+H_2\underrightarrow{t^o,Ni}CH_3-CH_2-CH_3\)
d, \(CH_3-C\equiv CH+AgNO_3+NH_3\rightarrow CH_3-C\equiv CAg_{\downarrow}+NH_4NO_3\)
e, \(CH_3-CH_2-CH_3+Cl_2\underrightarrow{as}CH_3-CHCl-CH_3+HCl\)
f, \(CH_2=CH-CH_3+H_2O\underrightarrow{t^o,xt}CH_3-CH\left(OH\right)-CH_3\)
g, \(CH\equiv CH+H_2O\underrightarrow{t^o,xt}CH_3CHO\)
h, \(CH_2=CH-CH_3+HCl\underrightarrow{t^o,xt}CH_3-CHCl-CH_3\)

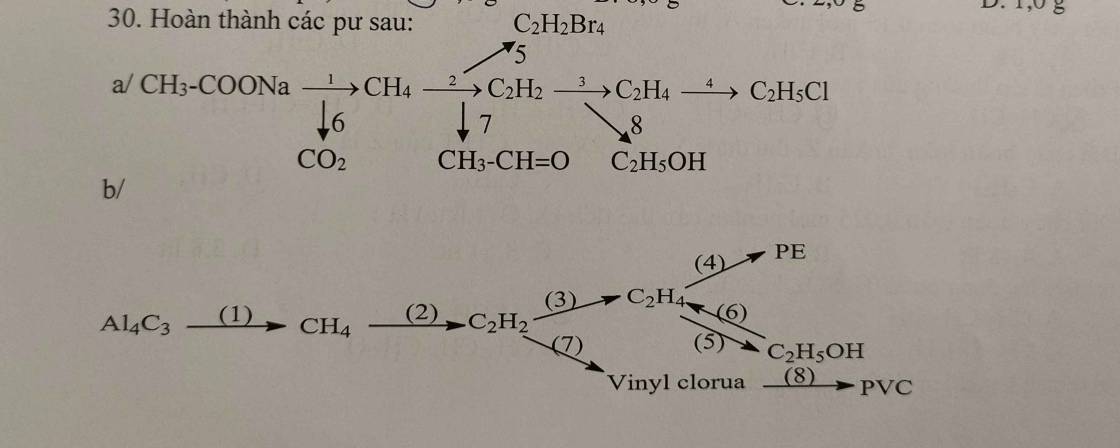
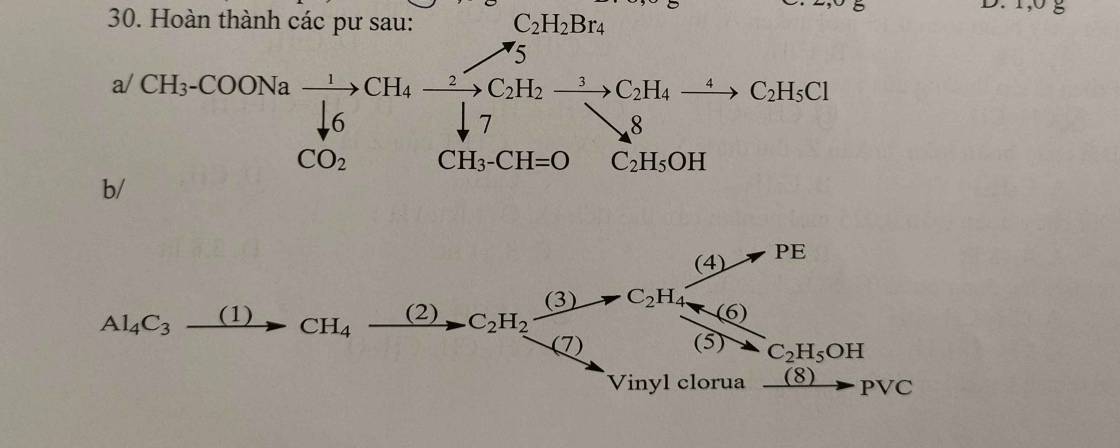
a, (1) \(CH_3COONa+NaOH\underrightarrow{^{t^o,CaO}}CH_4+Na_2CO_3\)
(2) \(2CH_4\underrightarrow{^{1500^oC,lln}}C_2H_2+3H_2\)
(3) \(C_2H_2+H_2\underrightarrow{^{t^o,Pd}}C_2H_4\)
(4) \(C_2H_4+H_2O\rightarrow C_2H_5OH\)
(5) \(CH\equiv CH+HCl\rightarrow CH_2=CHCl\)
(6) \(nCH_2=CHCl\underrightarrow{^{t^o,xt,p}}\left(-CH_2-CHCl-\right)_n\)
b, (1) \(CaC_2+2H_2O\rightarrow Ca\left(OH\right)_2+C_2H_2\)
(2) \(C_2H_2+H_2\underrightarrow{^{t^o,Pd}}C_2H_4\)
(3) \(nCH_2=CH_2\underrightarrow{^{t^o,xt,p}}\left(-CH_2-CH_2-\right)_n\)
(4) \(C_2H_2+2H_2\underrightarrow{^{t^o,Ni}}C_2H_6\)
(5) \(C_2H_6\underrightarrow{^{crackinh}}C_2H_4+H_2\)
(6) \(C_2H_6+Cl_2\underrightarrow{as}C_2H_5Cl+HCl\)

a, \(C_2H_6+Cl_2\xrightarrow[1:1]{as}C_2H_5Cl+HCl\)
b, \(nCH_2=CH-CH_3\underrightarrow{t^o,xt,p}\left(-CH_2-CH\left(CH_3\right)-\right)_n\)
c, \(CH_2=CH-CH_3+H_2\underrightarrow{t^o,Ni}CH_3CH_2CH_3\)
d, \(CH_3C\equiv CH+AgNO_3+NH_3\rightarrow CH_3C\equiv CAg_{\downarrow}+NH_4NO_3\)
e, \(CH_3-CH_2-CH_3+Cl_2\underrightarrow{as}CH_3-CHCl-CH_3+HCl\)
f, \(CH_2=CH-CH_3+H_2O\underrightarrow{t^o,xt}CH_3-CH\left(OH\right)-CH_3\)
g, \(CH\equiv CH+H_2O\underrightarrow{t^o,xt}CH_3-CHO\)
h, \(CH_2=CH-CH_3+HCl\underrightarrow{t^o,xt}CH_3-CHCl-CH_3\)
26)
a) \(C_2H_6+Cl_2\xrightarrow[]{a/s}C_2H_5Cl+HCl\)
b) \(nCH_2=CH-CH_3\xrightarrow[]{t^o,p,xt}\left(-CH_2-CH\left(CH_3\right)-\right)_n\)
c) \(CH_2=CH-CH_3+H_2\xrightarrow[]{Ni,t^o}CH_3-CH_2-CH_3\)
d) \(CH_3-C\equiv CH+AgNO_3+NH_3\rightarrow CH_3-C\equiv CAg\downarrow+NH_4NO_3\)
e) \(CH_3-CH_2-CH_3+Cl_2\xrightarrow[]{a/s}CH_3-CH_2-CH_2Cl+HCl\)
f) \(CH_2=CH-CH_3+H_2O\xrightarrow[]{t^o,xt}CH_3-CH\left(OH\right)-CH_3\)
g) \(CH\equiv CH+H_2O\xrightarrow[]{Hg^{2+},t^o}CH_3CHO\)
h) \(CH_2=CH-CH_3+HCl\xrightarrow[]{t^o}CH_3-CHCl-CH_3\)

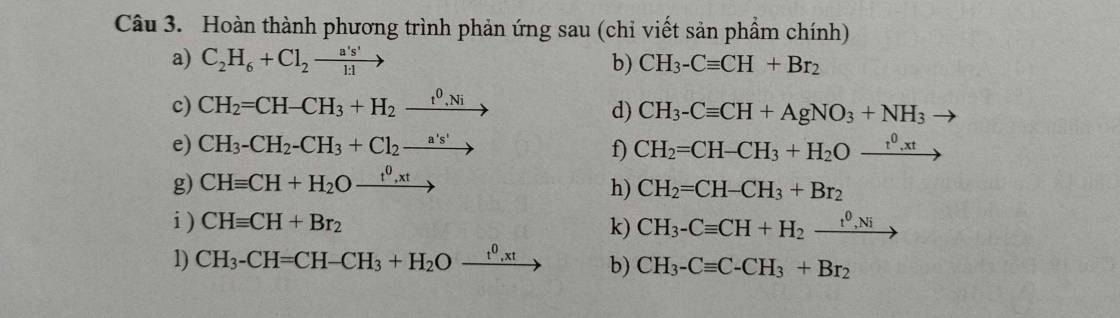
33)
a) \(C_2H_6+Cl_2\xrightarrow[]{a/s}C_2H_5Cl+HCl\)
b) \(CH_2=CH-CH_3\xrightarrow[]{t^o,xt}\left[{}\begin{matrix}CH\equiv C-CH_3+H_2\\CH_2=C=CH_2+H_2\end{matrix}\right.\)
c) \(CH_2=CH-CH_3+H_2\xrightarrow[]{t^o,xt}CH_3-CH_2-CH_3\)
d) \(CH_3-C\equiv CH+AgNO_3+NH_3\rightarrow CH_3-C\equiv CAg\downarrow+NH_4NO_3\)
e) \(CH_3-CH_2-CH_3+Cl_2\xrightarrow[]{a/s}CH_3-CH_2-CH_3Cl+HCl\)
f) \(CH_2=CH-CH_3+H_2O\xrightarrow[]{t^o,xt}\left[{}\begin{matrix}CH_3-CH\left(OH\right)-CH_3\left(sp.ch\text{ính}\right)\\OH-CH_2-CH_2-CH_3\left(sp.ph\text{ụ}\right)\end{matrix}\right.\)
g) \(CH\equiv CH+H_2O\xrightarrow[]{t^o,xt}CH_3CHO\)
h) \(CH_2=CH-CH_3+HCl\xrightarrow[]{t^o,xt}\left[{}\begin{matrix}CH_3-CHCl-CH_3\left(sp.ch\text{ính}\right)\\CH_2Cl-CH_2-CH_3\left(sp.ph\text{ụ}\right)\end{matrix}\right.\)
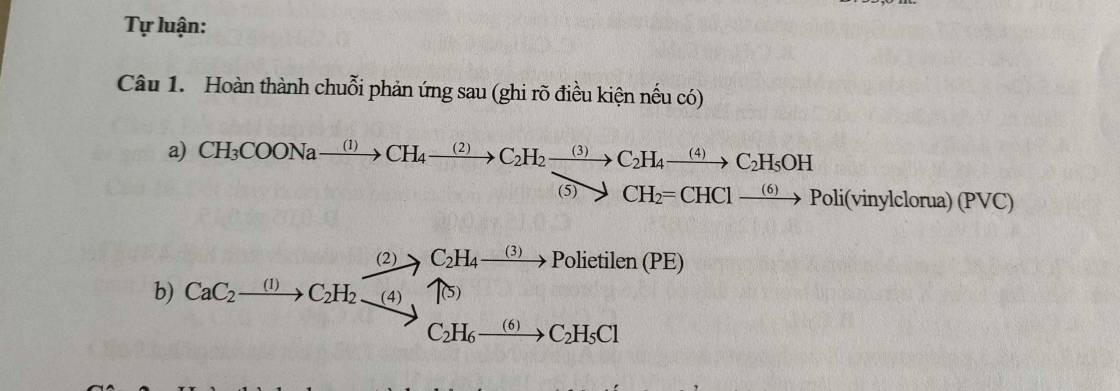
(1) \(CH_3COONa+NaOH\xrightarrow[]{CaO,t^o}CH_4\uparrow+Na_2CO_3\)
(2) \(2CH_4\xrightarrow[lln]{1500^oC}C_2H_2+3H_2\)
(3) \(C_2H_2+H_2\xrightarrow[]{Pd/PbCO_3,t^o}C_2H_4\)
(4) \(C_2H_4+HBr\xrightarrow[]{t^o,xt}C_2H_5Br\)
(5) \(2CH\equiv CH\xrightarrow[]{t^o,xt}CH_2=CH-C\equiv CH\)
(6) \(C_2H_2+2H_2\xrightarrow[]{Ni,t^o}C_2H_6\)


a, (1) \(CH_3COONa+NaOH\underrightarrow{t^o,CaO}Na_2CO_3+CH_4\)
(2) \(2CH_4\underrightarrow{^{1500^oC,lln}}C_2H_2+3H_2\)
(3)\(C_2H_2+H_2\underrightarrow{t^o,Pd}C_2H_4\)
(4)\(C_2H_4+HCl\underrightarrow{t^o,xt}C_2H_5Cl\)
(5) \(C_2H_2+2Br_2\rightarrow C_2H_2Br_4\)
(6) \(CH_4+2O_2\underrightarrow{t^o}CO_2+2H_2O\)
(7) \(C_2H_2+H_2O\underrightarrow{t^o,xt}CH_3CHO\)
(8) \(C_2H_4+H_2O\underrightarrow{t^o,xt}C_2H_5OH\)
b, (1) \(Al_4C_3+12H_2O\rightarrow4Al\left(OH\right)_3+3CH_4\)
(2) \(2CH_4\underrightarrow{^{1500^oC,lln}}C_2H_2+3H_2\)
(3) \(C_2H_2+H_2\underrightarrow{t^o,Pd}C_2H_4\)
(4) \(nCH_2=CH_2\underrightarrow{t^o,p,xt}\left(-CH_2-CH_2-\right)_n\)
(5) \(C_2H_4+H_2O\underrightarrow{t^o,xt}C_2H_5OH\)
(6) \(C_2H_5OH\xrightarrow[170^oC]{^{H_2SO_{4\left(đ\right)}}}C_2H_4+H_2O\)
(7)\(C_2H_2+HCl\underrightarrow{t^o,xt}C_2H_3Cl\)
(8) \(nCH_2=CHCl\underrightarrow{t^o}\left(-CH_2-CHCl-\right)_n\)
a.
\(\left(1\right)CH_3-CHOONa+NaOH\xrightarrow[CaO]{t^o}Na_2CO_3+CH_4\)
\(\left(2\right)2CH_4\xrightarrow[t^o]{làm.lạnh.nhanh}CH\equiv CH+3H_2\)
\(\left(3\right)CH\equiv CH+H_2\xrightarrow[Pd]{t^o}CH_2=CH_2\)
\(\left(4\right)CH_2=CH_2+HCl\rightarrow\left(askt\right)C_2H_5Cl\)
\(\left(5\right)CH\equiv CH+2Br_{2\left(dd\right)}\rightarrow CHBr_2-CHBr_2\)
\(\left(6\right)CH_4+2O_2\rightarrow\left(t^o\right)CO_2+2H_2O\)
\(\left(7\right)CH\equiv CH+H_2O\xrightarrow[HgCl_2]{t^o}CH_3-CH=O\)
\(\left(8\right)CH_2=CH_2+H_2O\xrightarrow[H_2SO_{4\left(đ\right)}]{t^o}C_2H_5OH\)
b.
\(\left(1\right)Al_4C_3+12H_2O\rightarrow4Al\left(OH\right)_3\downarrow+3CH_4\)
\(\left(2\right)2CH_4\xrightarrow[làm.lạnh.nhanh]{t^o}CH\equiv CH+3H_2\)
\(\left(3\right)CH\equiv CH+H_2\xrightarrow[Pd]{t^o}CH_2=CH_2\)
\(\left(4\right)nCH_2=CH_2\underrightarrow{t^o,xt,p}\left(-CH_2-CH_2-\right)n\)
\(\left(5\right)CH_2=CH_2+H_2O\xrightarrow[H_2SO_{4\left(đ\right)}]{t^o}C_2H_5OH\)
\(\left(6\right)C_2H_5OH\xrightarrow[H_2SO_{4\left(đ\right)}]{t^o}CH_2=CH_2+H_2O\)
\(\left(7\right)CH\equiv CH+HCl\xrightarrow[HgCl_2]{t^o}CH_2=CHCl\)
\(\left(8\right)nCH_2=CHCl\underrightarrow{t^o,xt,p}\left(-CH_2-CHCl\right)n\)

ta có:\(x^3+x^2+2x^2+2x+2x+2=0\)0
\(\Leftrightarrow x^2\left(x+1\right)+2x\left(x+1\right)+2\left(x+1\right)=0\)
\(\Leftrightarrow\left(x^2+2x+2\right)\left(x+1\right)=0\)
Do \(x^2+2x+2\ne0\)
\(\Rightarrow x+1=0\)
\(\Rightarrow x=-1\)
vậy phương trình trên có tập nghiệm là :S=(-1)



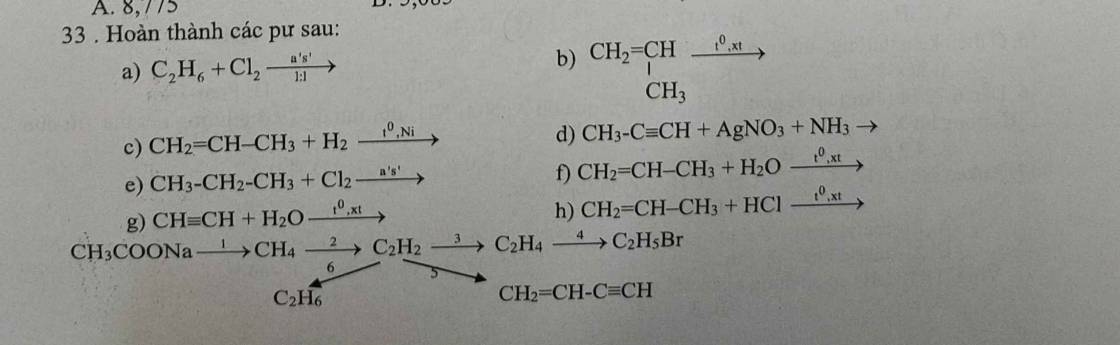
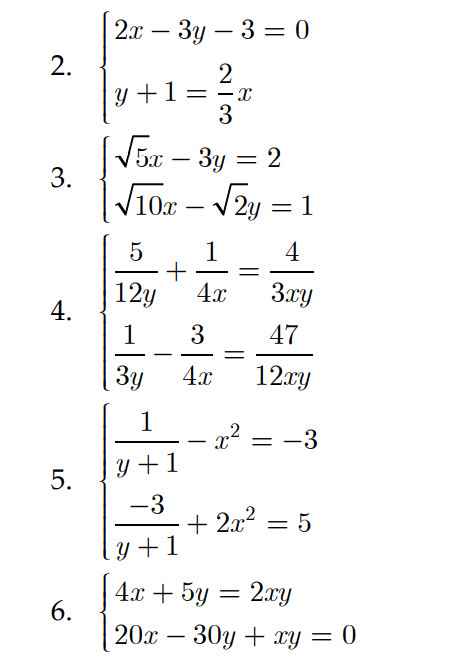
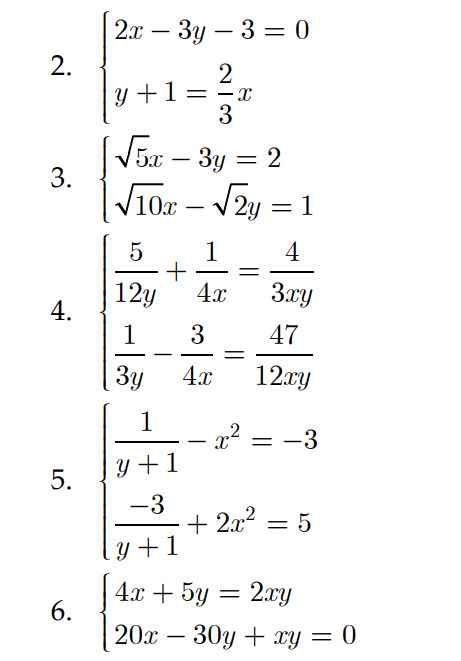
Mình đã trả lời ở câu hỏi này rồi bạn nhé.
https://hoc24.vn/cau-hoi/giup-minh-cac-phuong-trinh-nay-voi.7778830873437