Giải giúp e câu b với ạ. Em cần gấp
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


b: Tọa độ giao là:
5x-4=2x+2 và y=2x+2
=>x=2 và y=6
c: Vì (d2)//d nên (d2): y=2x+b
Thay x=1 và y=3 vào (d2), ta được:
b+2=3
=>b=1

2. There used to have many old buildings 10 years ago
3. I wish a new mall didn't build
4. I have had this wardrobe since my wedding day
5. She hasn't been seen for two years

Mary if she could speak some foreign languages
Lan if she was going to visit her aunt the day after
what I was doing
how she was feeling then
what I usually did in my free time
why he why he didn't come there to meet her
why I was so lazy and naughty
like playing soccer, don't you?
goes to school late, doesn't he?
can swim very well, can't you?
is going to the party, isn't she?
was published in Germany in 1550, wasn't it?
are sold all over the world, aren't they?
have been built this year, haven't they?
was given a book, wasn't he?
was bought by Mrs Brown yesterday, wasn't she?
is used every day, isn't it?
be beautiful sights in this village when I lived here

Ta có:
\(\dfrac{x}{10}=\dfrac{y}{5}\)
\(\Rightarrow\dfrac{x}{20}=\dfrac{y}{10}\) \(\left(1\right)\)
\(\dfrac{y}{2}=\dfrac{z}{3}\)
\(\Rightarrow\dfrac{y}{10}=\dfrac{z}{15}\) \(\left(2\right)\)
Từ \(\left(1\right)\) và \(\left(2\right)\)
\(\Rightarrow\dfrac{x}{20}=\dfrac{y}{10}=\dfrac{z}{15}\)
Lại có:
\(\dfrac{z}{15}=\dfrac{4z}{60}\)
Áp dụng tính chất của dãy tỉ số bằng nhau,ta có:
\(\dfrac{x}{20}=\dfrac{y}{10}=\dfrac{4z}{60}=\dfrac{x+4z}{20+60}=\dfrac{240}{80}=3\)
\(\Rightarrow x=3\cdot20=60\)
\(y=3\cdot10=30\)
\(z=3\cdot15=45\)




câu 6: bài học: hãy là chính mình, biết sống hết mình, làm tốt những trách nhiệm, bổn phận của bản thân sẽ được mọi người tôn trọng, yêu quý
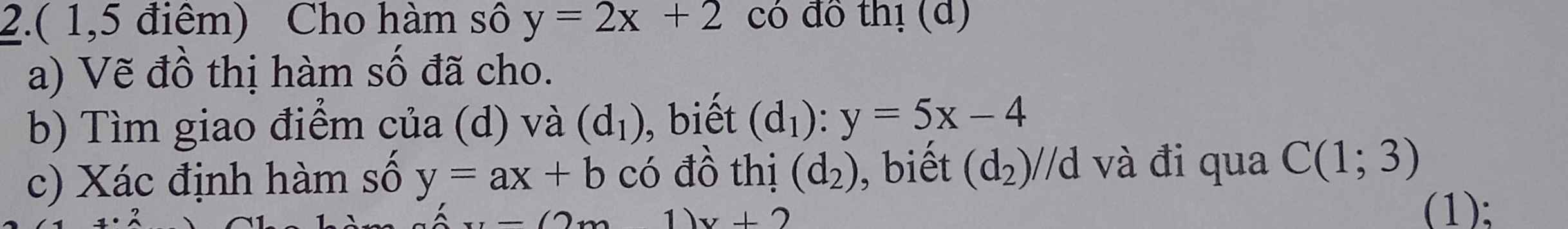



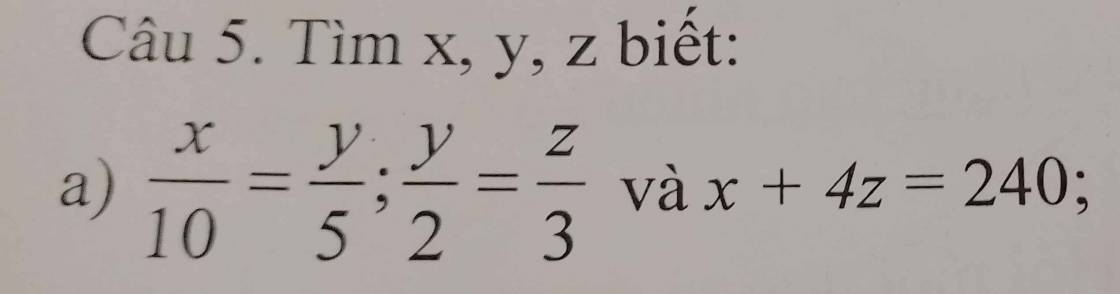




a)
4Na + O2 ---to→ 2Na2O
Na2O + H2O → 2NaOH
2NaOH + CO2 → Na2CO3 + H2O
Na2CO3 + Ca(OH)2 → 2NaOH + CaCO3
CaCO3 ---to→ CaO + CO2
CO2 + NaOH → NaHCO3
NaHCO3 + H2SO4 → Na2SO4 + CO2 + H2O
Na2SO4 + Ba(OH)2 → 2NaOH + BaSO4
b)
S + O2 ---to→ SO2
2SO2 + O2 ---to(V2O5)→ 2SO3
SO3 + H2O → H2SO4
H2SO4 + Cu(OH)2 → CuSO4 + 2H2O
CuSO4 + FeCl2 → CuCl2 + FeSO4
FeSO4 + 2NaOH → Fe(OH)2 + Na2SO4
Fe(OH)2 + 2HCl → FeCl2 + 2H2O
2FeCl2 + Cl2 → 2FeCl3
2FeCl3 + 3Ba(OH)2 → 2Fe(OH)3 + 3BaCl2
2Fe(OH)3 ---to→ Fe2O3 + 3H2O
Fe2O3 + 3H2SO4 → Fe2(SO4)3 + 3H2O