phân hiuyr hoàn toàn 61,25 gam KCIO3 thu được bao nhiêu lít khí oxi ở(đktc)biết(K=39,Cl=35,5;O=16)
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


\(n_{KClO_3}=\dfrac{12,25}{122,5}=0,1mol\)
\(2KClO_3\rightarrow\left(t^o,MnO_2\right)2KCl+3O_2\)
0,1 0,15 ( mol )
\(V_{O_2}=0,15.22,4=3,36l\)
nKClO3 = 12,25 : 122,5 = 0,1 (mol)
pthh : 2KClO3 -t--> 2KCl + 3O2
0,1 --------------------->0,15 (mol)
=> VO2(dktc) = 0,15 . 22,4 = 3,36 ( l)

nO2=6,72/22,4=0,3 mol
PTPƯ: 2KClO3 Nhiệt Phân→ 2KCl + 3O2↑
0,3 mol O2 ---> 0,2 mol KClO3
nên mKClO3=122,5.0,2=24,5 g

Ta có: \(n_{KClO_3}=\dfrac{49}{122,5}=0,4\left(mol\right)\)
PT: \(2KClO_3\underrightarrow{t^o}2KCl+3O_2\)
______0,4______0,4_____0,6 (mol)
\(\Rightarrow m_{KCl}=0,4.74,5=29,8\left(g\right)\)
\(V_{O_2}=0,6.22,4=13,44\left(l\right)\)

\(PTHH:2KClO_3\underrightarrow{t^o}2KCl+3O_2\\ n_{O_2}=\dfrac{V_{\left(đktc\right)}}{22,4}=\dfrac{67,2}{22,4}=3\left(mol\right)\\ Theo.PTHH:n_{KClO_3}=\dfrac{2}{3}.n_{O_2}=\dfrac{2}{3}.3=2\left(mol\right)\\ m_{KClO_3}=n.M=2.122,5=245\left(g\right)\)

a) \(n_{O_2}=\dfrac{11,2}{22,4}=0,5\left(mol\right)\)
PTHH: 2KMnO4 --to--> K2MnO4 + MnO2 + O2
1<-----------------------------0,5
=> \(m_{KMnO_4}=1.158=158\left(g\right)\)
b) \(n_{Fe_2O_3}=\dfrac{80}{160}=0,5\left(mol\right)\)
PTHH: Fe2O3 + 3H2 --to--> 2Fe + 3H2O
0,5--->1,5
=> \(V_{H_2}=1,5.22,4=33,6\left(l\right)\)
c) \(n_{CuO}=\dfrac{8}{80}=0,1\left(mol\right)\)
\(n_{H_2}=\dfrac{1,12}{22,4}=0,05\left(mol\right)\)
PTHH: CuO + H2 --to--> Cu + H2O
Xét tỉ lệ: \(\dfrac{0,1}{1}>\dfrac{0,05}{1}\) => CuO dư, H2 hết
PTHH: CuO + H2 --to--> Cu + H2O
0,05<-0,05---->0,05-->0,05
=> \(n_{Cu\left(dư\right)}=0,1-0,05=0,05\left(mol\right)\)
mCu = 0,05.64 = 3,2 (g)
VH2O = 0,05.22,4 = 1,12 (l)
a)\(n_{O_2}=\dfrac{11,2}{22,4}=0,5mol\)
\(2KMnO_4\underrightarrow{t^o}K_2MnO_4+MnO_2+O_2\)
1 0,5
\(M_{KMnO_4}=1\cdot158=158g\)
b)\(n_{Fe_2O_3}=\dfrac{80}{160}=0,5mol\)
\(Fe_2O_3+3H_2\rightarrow2Fe+3H_2O\)
0,5 1,5
\(V_{H_2}=1,15\cdot22,4=25,76l\)

a.\(n_{Cu}=\dfrac{6,4}{64}=0,1mol\)
\(2Cu+O_2\rightarrow\left(t^o\right)2CuO\)
0,1 0,05 ( mol )
\(V_{kk}=\left(0,05.22,4\right).5=5,6l\)
b.\(2KClO_3\rightarrow\left(t^o\right)2KCl+3O_2\)
1/30 0,05 ( mol )
\(m_{KClO_3}=\dfrac{1}{30}.122,5=4,08g\)

nO2=6,72/22,4=0,3(mol)
PTHH: 2 KClO3 -to->2 KCl +3 O2
Ta có: nKClO3=2/3. 0,3=0,2(mol)
=>mKClO3=0,2.122,5=24,5(g)

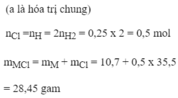
\(n_{KClO_3}=\dfrac{61,25}{122,5}=0,5mol\)
\(2KClO_3\rightarrow\left(t^o,MnO_2\right)2KCl+3O_2\)
0,5 0,75 ( mol )
\(V_{O_2}=0,75.22,4=16,8l\)