Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(a,n_{C_6H_{12}O_6}=\dfrac{36}{180}=0,2\left(mol\right)\)
PTHH: \(C_6H_{12}O_6\underrightarrow{\text{men rượu}}2C_2H_5OH+2CO_2\uparrow\)
0,2----------------->0,4----------->0,4
=> VCO2 = 0,4.22,4 = 8,96 (l)
b, mC2H5OH = 0,4.46.50% = 9,2 (g)
\(c,V_{C_2H_5OH}=\dfrac{9,2}{0,8}=11,5\left(ml\right)\\ \rightarrow V_{ddC_2H_5OH}=\dfrac{11,5.100}{60}=\dfrac{115}{6}\left(ml\right)\)

\(a) V_{rượu} = 5.\dfrac{10}{100} = 0,5(lít)\\ b) m_{rượu} = D.V = 0,78.0,5.1000 = 390(gam)\\ c) n_{C_2H_5OH\ pư} = \dfrac{380}{46}.80\% = \dfrac{156}{23}(mol)\\ C_2H_5OH + O_2 \xrightarrow{men\ giấm} CH_3COOH + H_2O\\ n_{CH_3COOH} = n_{C_2H_5OH\ pư} = \dfrac{156}{23}(mol)\\ m_{CH_3COOH} = \dfrac{156}{23}.60 = 406,96(gam)\)

Đổi 10kg = 10000g
Ta có: \(n_{CH_3COOH\left(LT\right)}=\dfrac{10000.5\%}{92\%}=\dfrac{12500}{23}\left(mol\right)\)
PTHH:
\(C_2H_5OH+O_2\xrightarrow[]{\text{men giấm}}CH_3COOH+H_2O\)
\(\dfrac{12500}{23}\)<---------------------\(\dfrac{12500}{23}\)
\(\Rightarrow m_{C_2H_5OH}=\dfrac{12500}{23}.46=25000\left(g\right)=25\left(kg\right)\)

a) C2H5OH + O2 --men giấm--> CH3COOH + H2O
b) \(n_{O_2}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\)
PTHH: C2H5OH + O2 --men giấm--> CH3COOH + H2O
0,3<----0,3------------------>0,3
=> \(m_{C_2H_5OH}=0,3.46=13,8\left(g\right)\)
=> \(V_{C_2H_5OH}=\dfrac{13,8}{0,8}=17,25\left(ml\right)\)
=> \(Độ.rượu=\dfrac{17,25}{150}.100=11,5^o\)
c) \(m_{CH_3COOH}=0,3.60=18\left(g\right)\)

a, n\(C_2H_6O\)= \(\frac{6,9}{46}\)=0,15mol
pt : C2H6O + 3O2 → 2CO2 + 3 H2O
(mol) 0,15mol → 0,45mol→0,3mol
m\(CO_2\) = 0,3 . 44 = 13,2 G
b, Vkk = \(\frac{20}{100}\) : (0,45 . 22,4 ) = 50,4l

Bài 1:
PTHH: \(C_2H_5OH+O_2\xrightarrow[]{mengiấm}CH_3COOH+H_2O\)
Ta có: \(n_{C_2H_5OH}=\dfrac{115\cdot0,8}{46}=2\left(mol\right)=n_{CH_3COOH\left(lýthuyết\right)}\)
\(\Rightarrow m_{CH_3COOH\left(thực\right)}=2\cdot60\cdot90\%=108\left(g\right)\)
Bài 2:
PTHH: \(C_2H_5OH+CH_3COOH\xrightarrow[H_2SO_4\left(đ\right)]{t^o}CH_3COOC_2H_5+H_2O\)
Ta có: \(\left\{{}\begin{matrix}n_{CH_3COOH}=\dfrac{60}{60}=1\left(mol\right)\\n_{C_2H_5OH}=\dfrac{92}{46}=2\left(mol\right)\end{matrix}\right.\) \(\Rightarrow\) Rượu còn dư, Axit p/ứ hết
\(\Rightarrow n_{CH_3COOC_2H_5\left(lýthuyết\right)}=1\left(mol\right)\) \(\Rightarrow m_{CH_3COOC_2H_5\left(thực\right)}=1\cdot88\cdot80\%=70,4\left(g\right)\)

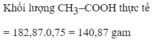
\(C_2H_5OH+O_2\rightarrow\left(men.giấm\right)CH_3COOH+H_2O\\ n_{CH_3COOH}=\dfrac{200.12\%}{60}=0,4\left(mol\right)\\ n_{C_2H_5OH}=n_{CH_3COOH}=0,4\left(mol\right)\\ m_{C_2H_5OH}=0,4.46=18,4\left(g\right)\\ V_{C_2H_5OH}=\dfrac{18,4}{0,8}=23\left(ml\right)\)
Ta có: \(m_{CH_3COOH}=200.12\%=24\left(g\right)\Rightarrow n_{CH_3COOH}=\dfrac{24}{60}=0,4\left(mol\right)\)
PT: \(C_2H_5OH+O_2\underrightarrow{^{mengiam}}CH_3COOH+H_2O\)
Theo PT: \(n_{C_2H_5OH}=n_{CH_3COOH}=0,4\left(mol\right)\)
\(\Rightarrow m_{C_2H_5OH}=0,4.46=18,4\left(g\right)\)
\(\Rightarrow V_{C_2H_5OH}=\dfrac{18,4}{0,8}=23\left(ml\right)\)