Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{Al_2O_3}=\dfrac{5.1}{102}=0.05\left(mol\right)\)
\(Al_2O_3+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\)
\(0.05...........0.15...............0.05\)
\(C_{M_{H_2SO_4}}=\dfrac{0.15}{0.2}=0.75\left(M\right)\)
\(C_{M_{Al_2\left(SO_4\right)_3}}=\dfrac{0.05}{0.2}=0.25\left(M\right)\)

Bài 1:
\(a.n_{NaOH\left(tổng\right)}=0,05.1+0,2.0,2=0,09\left(mol\right)\\ V_{ddNaOH\left(tổng\right)}=50+200=250\left(ml\right)=0,25\left(l\right)\\ C_{MddNaOH\left(cuối\right)}=\dfrac{0,09}{0,25}=0,36\left(M\right)\\ b.n_{HCl}=0,5.0,02=0,01\left(mol\right)\\ n_{H_2SO_4}=0,08.0,2=0,016\left(mol\right)\\ V_{ddsau}=20+80=100\left(ml\right)=0,1\left(l\right)\\ C_{MddH_2SO_4}=\dfrac{0,016}{0,1}=0,16\left(M\right)\\ C_{MddHCl}=\dfrac{0,01}{0,1}=0,1\left(M\right)\)
Bài 2:
\(a.m_{H_2SO_4}=29,4.10\%=2,94\left(g\right)\\ b.n_{H_2SO_4}=\dfrac{2,94}{98}=0,03\left(mol\right)\\ n_{Fe}=\dfrac{0,56}{56}=0,01\left(mol\right)\\ Fe+H_2SO_4\rightarrow FeSO_4+H_2\\ Vì:\dfrac{0,01}{1}< \dfrac{0,03}{1}\Rightarrow H_2SO_4dư\\ n_{H_2SO_4\left(dư\right)}=0,03-0,01=0,02\left(mol\right)\\ m_{H_2SO_4\left(dư\right)}=0,02.98=1,96\left(g\right)\\ n_{H_2}=n_{Fe}=0,01\left(mol\right)\\ \Rightarrow V_{H_2\left(đktc\right)}=0,01.22,4=0,224\left(l\right)\)

MgCl2+2AgNO3->Mg(NO3)2+2AgCl
0,04-----0,08-----------0,04----------0,08
n MgCl2=0,1 mol
n AgNO3=0,08 mol
=>Mgcl2 dư
=>m AgCl=0,08.143,5=11,48g
=>CMMg(NO)2=\(\dfrac{0,04}{0,2}\)=0,2M
=>CMMgcl2 dư=\(\dfrac{0,06}{0,2}\)=0,3M
\(n_{MgCl_2}=0,1\cdot1=0,1mol\)
\(n_{AgNO_3}=0,1\cdot0,8=0,08mol\)
\(MgCl_2+2AgNO_3\rightarrow2AgCl\downarrow+Mg\left(NO_3\right)_2\)
0,1 0,08 0 0
0,04 0,08 0,08 0,04
0,06 0 0,08 0,04
\(m_{\downarrow}=0,08\cdot143,5=11,48g\)
\(C_{M_{Mg\left(NO_3\right)_2}}=\dfrac{n_{Mg\left(NO_3\right)_2}}{V_X}=\dfrac{0,04}{0,2}=0,2M\)

Bài 1:
\(n_{KNO_3}=\dfrac{20}{101}=0,198\left(mol\right)\)
\(C_M=\dfrac{n}{V}=\dfrac{0,198}{0,85}=0,233M\)
Bài 2:
\(C_M=\dfrac{n}{V}=\dfrac{0,5}{0,75}=0,66M\)
Bài 3:
\(n_{KNO_3}=2.0,5=1\left(mol\right)\)
\(m_{KNO_3}=1.101=101\left(g\right)\)
Bài 4:
\(C\%=\dfrac{20}{600}.100=3,33\%\)
Bài 1:
\(n_{KNO_3}=\dfrac{20}{101}=0,198\left(mol\right)\)
\(C_{M_{ddKNO_3}}=\dfrac{0,198}{0,85}\approx0,23M\)
Bài 2:
\(C_{M_{ddKCl}}=\dfrac{0,5}{0,75}\approx0,667M\)
Bài 3:
\(n_{KNO_3}=0,5.2=1\left(mol\right)\Rightarrow m_{KNO_3}=1.101=101\left(g\right)\)
Bài 4:
\(C\%_{ddKCl}=\dfrac{20.100\%}{600}=3,333\%\)

\(n_{NaOH}=0,5.0,2=0,1\left(mol\right);n_{HCl}=0,5.0,3=0,15\left(mol\right)\)
PTHH: NaOH + HCl → NaCl + H2O
Mol: 0,1 0,1 0,1
Ta có: \(\dfrac{0,1}{1}< \dfrac{0,15}{1}\) ⇒ NaOH hết, HCl dư
Vdd sau pứ = 0,2 + 0,3 = 0,5 (l)
\(C_{M_{ddNaCl}}=\dfrac{0,1}{0,5}=0,2M\)
\(C_{M_{ddHCldư}}=\dfrac{0,15-0,1}{0,5}=0,1M\)

Bài tập 4:
Số mol :
\(n_{MgO}=\dfrac{6}{40}=0,15mol\)
PHHH:
\(MgO\) + \(H_2SO_4\) ---> \(MgSO_4\) + \(H_2O\)
0,15 0,15 0,15 0,15
a,Theo phương trình :
\(n_{H_2SO_4}=0,15\Rightarrow m_{H_2SO_4}=0,15.98=14,7g\)b,
Ta có :
\(m_{ddH_2SO_4}=D.V=1,2.50=60\left(g\right)\)
\(\Rightarrow\) Nồng độ % của \(H_2SO_4\) là :
\(C\%_{ddH_2SO_4}=\dfrac{14,7}{60}.100\%=24,5\%\)
c, Theo phương trình :
\(n_{MgSO_4}=0,15\Rightarrow m_{MgSO_4}=0,15.120=18g\)Khối lượng dung dịch sau khi phản ứng là :
\(m_{ddsau}=m_{MgO}+m_{ddH_2SO}_{_4}=60+6=66g\)Nồng độ % dung dịch sau phản ứng là :
\(C\%_{ddsau}=\dfrac{18}{66}.100\%=27,27\%\)
Bài tập 4 :
Theo đề bài ta có :
nMgO=6/40=0,15(mol)
mddH2SO4=V.D=50.1,2=60(g)
ta có pthh :
MgO + H2SO4 \(\rightarrow\) MgSO4 + H2O
0,15mol...0,15mol...0,15mol
a) Khối lượng axit H2SO4 đã phản ứng là :
mH2SO4=0,15.98=14,7 g
b) Nồng độ % của dd axit là :
C%ddH2SO4=\(\dfrac{14,7}{60}.100\%=24,5\%\)
c) Nồng độ % của dung dịch sau p/ư là :
Ta có :
mct=mMgSO4=0,15.120=18 g
mddMgSO4=6 + 60 = 66 g
=> C%ddMgSO4=\(\dfrac{18}{66}.100\%\approx27,273\%\)
Vậy....

Bài 1:
nH2SO4 bđ = 0,5 . 0,2 = 0,1 mol
nH2 = \(\dfrac{1,792}{22,4}=0,08\left(mol\right)\)
Pt: Zn + H2SO4 --> ZnSO4 + H2
............0,08 mol<-0,08 mol<-0,08 mol
Theo pt: nH2SO4 pứ = nH2 = 0,08 mol < 0,1 mol
=> HCl dư
CM H2SO4 dư = \(\dfrac{\left(0,1-0,08\right)}{0,2}=0,1M\)
CM ZnSO4 = \(\dfrac{0,08}{0,2}=0,4M\)
Bài 2:
nCuO = \(\dfrac{3,2}{80}=0,04\left(mol\right)\)
mH2SO4 = \(\dfrac{150\times32,666}{100}=49\left(g\right)\)
nH2SO4 = \(\dfrac{49}{98}=0,5\left(mol\right)\)
Pt: CuO + H2SO4 --> CuSO4 + H2O
0,04 mol->0,04 mol->0,04 mol
Xét tỉ lệ mol giữa CuO và H2SO4:
\(\dfrac{0,04}{1}< \dfrac{0,5}{1}\)
Vậy H2SO4 dư
mH2SO4 dư = (0,5 - 0,04) . 98 = 45,08 (g)
mCuSO4 = 0,04 . 160 = 6,4 (g)
mdd sau pứ = mCuO + mdd H2SO4 = 3,2 + 150 = 153,2 (g)
C% dd CuSO4 = \(\dfrac{6,4}{153,2}.100\%=4,177\%\)
C% dd H2SO4 dư = \(\dfrac{45,08}{153,2}.100\%=29,425\%\)
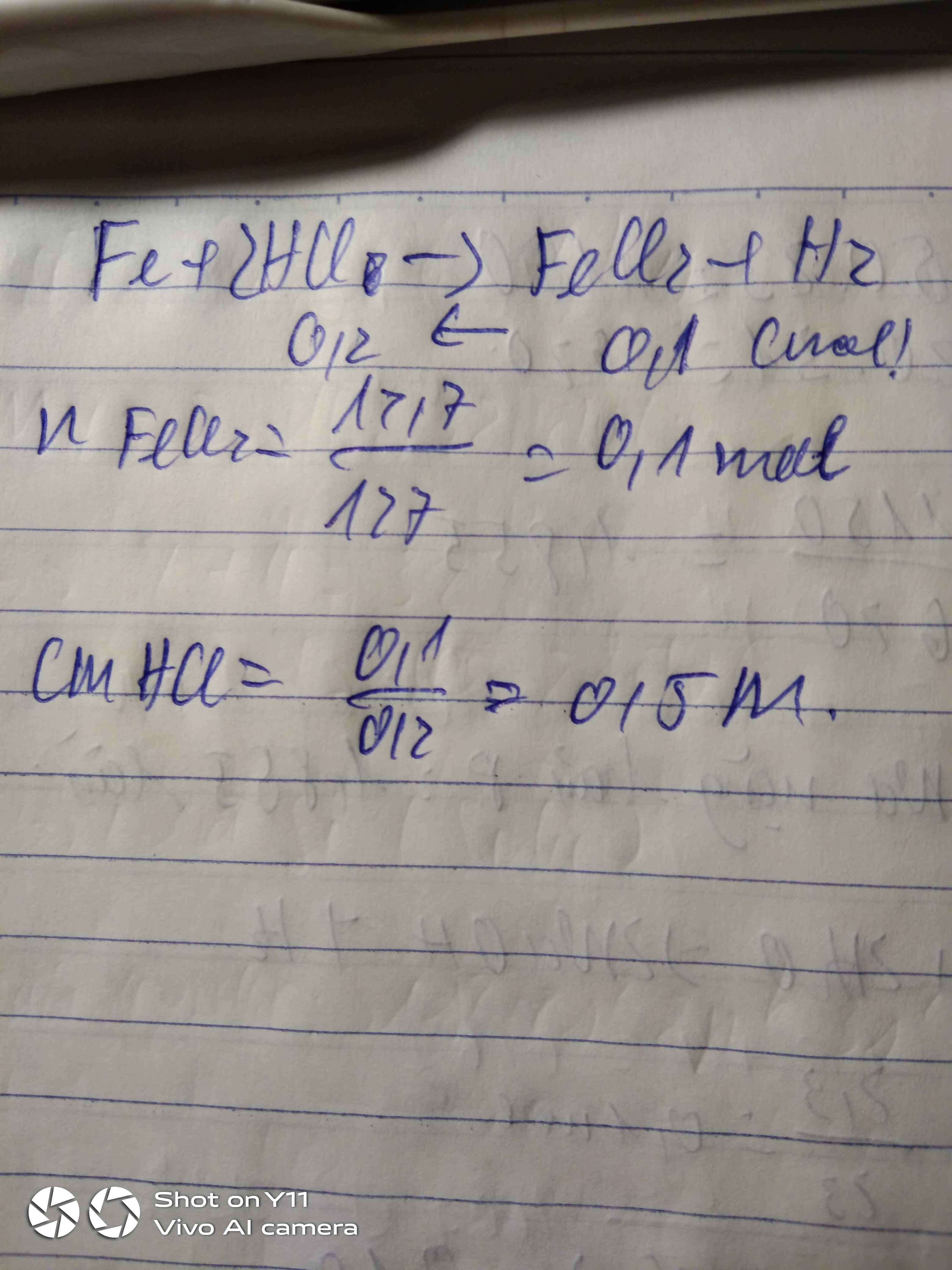
a)m Al2(SO4)3=6,84.\(\dfrac{200}{100}\)=13,68g
=>n Al2(SO4)3=0,04 mol
b)n HCl=3.0,2=0,6 mol
a. \(m_{Al_2\left(SO_4\right)_3}=200.6,84\%=13,68\left(g\right)\)
\(n_{Al_2\left(SO_4\right)_3}=\dfrac{13.68}{342}=0,04\left(mol\right)\)
b. \(n_{HNO_3}=0,2.3=0,6\left(mol\right)\)