Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(a.m_{Mg}=0,1.24=2,4\left(g\right)\\ m_{Ca}=0,2.40=8\left(g\right)\\ b.n_{hh}=\dfrac{2,8}{28}+\dfrac{13,2}{44}=0,4\left(mol\right)\\ \Rightarrow V_{hh}=0,4.22,4=8.96\left(l\right)\)

\(n_{O_2}=\dfrac{3,2}{32}=0,1\left(mol\right)\)
=> Vhh = (0,1+0,15).22,4 = 5,6 (l)

a)mCuO=0.25*(64+16)=20(g)
b)\(n_{MgCl_2}=\dfrac{19}{95}=0.2\left(mol\right)\)
Số phân từ MgCl2 có trong 19g là
0.2*6*1023=1,2.1023
c)
\(V_{hh}=\left(0.2+0.3+\dfrac{6.4}{32}\right).22,4=\left(0.5+0.2\right)=0.7\cdot22,4=15,68\left(l\right)\)


a, VO\(_2\) = 0,15 . 22,4 = 3,36 lít
b, V\(CO_2\) = \((\dfrac{48}{44}).22,4\approx24,43\) ( lít )
c, \(V_{SO_2}=\left(\dfrac{16}{64}\right).22,4=5,6\) ( lít )
\(V_{H_2}=\left(\dfrac{18.10^{23}}{6.10^{23}}\right).22,4=67,2\) ( lít )
=> \(V_{hh}=5,6+67,2=72,8\) ( lít )

\(n_{Cl_2}=a\left(mol\right)\)
\(n_{O_2}=b\left(mol\right)\)
\(n_Y=a+b=\dfrac{5.6}{22.4}=0.25\left(mol\right)\left(1\right)\)
\(m_Y=71a+32b=12.8\left(g\right)\left(2\right)\)
\(\left(1\right),\left(2\right):a=\dfrac{8}{65},b=\dfrac{33}{260}\)

1. \(n_{O_2}=\frac{V}{22,4}=\frac{6,72}{22,4}=0,3\left(mol\right)\)
2.
\(n_{CO_2}=\frac{m}{M}=\frac{4,4}{44}=0,1\left(mol\right)\)
\(n_{O_2}=\frac{m}{M}=\frac{3,2}{32}=0,1\left(mol\right)\)
\(V_{HC}=n.22,4=\left(0,1+0,1\right).22,4=4,48\left(l\right)\)
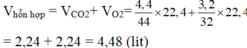

\(n_{O_2}=\dfrac{12,8}{32}=0,4\left(mol\right)\\ n_{CO_2}=\dfrac{13,2}{44}=0,3\left(mol\right)\\ \Rightarrow V_{hh}=\left(0,4+0,3\right).22,4=15,68\left(l\right)\)