Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(1) n_{KMnO_4}= \dfrac{31,6}{158} = 0,2(mol)\\ 2KMnO_4 \xrightarrow{t^o} K_2MnO_4 + MnO_2 + O_2\\ n_{O_2} = \dfrac{1}{2}n_{KMnO_4} = 0,1(mol)\\ V_{O_2} = 0,1.22,4 = 2,24(lít)\\ 2) CH_4 + 2O_2 \xrightarrow{t^o} CO_2 + 2H_2O\\ V_{CH_4} = \dfrac{1}{2}V_{O_2} = 1,12(lít)\\ 3)n_{CH_4} = \dfrac{1,12}{22,4} = 0,05(mol)\\ \text{Nhiệt lượng tỏa ra = } = 0,05.880 = 44(KJ)\)

a)\(n_{Fe}=\dfrac{22,4}{56}=0,4mol\)
\(3Fe+2O_2\underrightarrow{t^o}Fe_3O_4\)
0,4 \(\dfrac{4}{15}\) \(\dfrac{2}{15}\)
\(V_{O_2}=\dfrac{4}{15}\cdot22,4=5,973l\)
b)\(2KClO_3\underrightarrow{t^o}2KCl+3O_2\)
\(\dfrac{8}{45}\) \(\dfrac{4}{15}\)
\(m_{KClO_3}=\dfrac{8}{45}\cdot122,5=21,78g\)

a) 3Fe+2O2--->FE3O4
0,5------1/3 (mol)
4Al+3O2---.2Al2O3
1,25--0,9375(mol)
2Zn+O2--->2ZnO
1,5---0,75(mol)
n O2=1/3+0,9375+0,75=2,02(mol)
m O2=2,02.32=64,64(g)
b) 4P+5O2-->2P2O5
0,1-----0,125(mol)
S+02--->SO2
0,2--0,2(mol)
C+O2-->CO2
0,3--0,3(mol)
n O2=0,125+0,2+0,3=0,625(mol)
m O2=0,625.32=20(g)
c) n CH4=1,6/16=0,1(mol)
n CO=2,8/28=0,1(mol)
n C4H10=0,58/58=0,01(mol)
CH4+2O2--->CO2+2H2O
0,1---0,2(mol)
2CO+O2-->2CO2
0,1--0,05(mol)
C4H10+13/2O2--->4CO2+5H2O
0,01-----0,065(mol)
n O2=0,2+0,05+0,065=0,315(mol)
m O2=0,315.32=10,08(g)

a. \(2KMnO_4\rightarrow K_2MnO_4+MnO_2+O_2\)
\(n_{KMnO_4}=0,6mol\)
\(\rightarrow n_{O_2}=\frac{1}{2}n_{KMnO_4}=0,3mol\)
\(\rightarrow V_{O_2}=6,72l\)
\(V_{O_2\text{thực}}=\frac{6,72.75}{100}=5,04l\)
b. \(2KMnO_4\rightarrow K_2MnO_4+MnO_2+O_2\)
\(n_{O_2}=1,5mol\)
\(\rightarrow n_{KMnO_4}=2n_{O_2}=3mol\)
\(\rightarrow m_{KMnO_4\text{cần}}=\frac{474.100}{80}=592,5g\)

Bạn lập các PTHH sau đó chuyển tất cả thành số mol.Tính số mol của Oxi theo các chất đã cho

a) Gọi số mol Al, Zn là 2a, a (mol)
PTHH: 4Al + 3O2 --to--> 2Al2O3
2a-->1,5a---------->a
2Zn + O2 --to--> 2ZnO
a---->0,5a------->a
=> \(102a+81a=18,3\)
=> a = 0,1 (mol)
=> \(\left\{{}\begin{matrix}\%m_{Al}=\dfrac{0,2.27}{0,2.27+0,1.65}.100\%=45,378\%\\\%m_{Zn}=\dfrac{0,1.65}{0,2.27+0,1.65}.100\%=54,622\%\end{matrix}\right.\)
b) \(n_{O_2}=1,5a+0,5a=0,2\left(mol\right)\)
=> \(V_{O_2}=0,2.22,4=4,48\left(l\right)\)

a. \(n_{Fe}=0,5.56=28\left(g\right)\)
\(3Fe+2O_2\rightarrow Fe_3O_4\)
\(n_{O2}=\frac{2}{3}n_{Fe}=\frac{2}{3}.0,5=0,333\left(mol\right)\)
\(\Rightarrow V_{O2}=0,333.22,4=7,46\left(l\right)\)
\(4Al+3O_2\rightarrow2Al_2O_3\)
\(n_{O2}=\frac{3}{4}n_{Al}=\frac{3}{4}.1,25=0,9375\left(mol\right)\)
\(\Rightarrow V_{O2}=0,9375.22,4=21\left(l\right)\)
\(2Zn+O_2\rightarrow2ZnO\)
\(n_{O2}=\frac{1}{2}n_{Zn}=\frac{1}{2}.1,5=0,75\left(mol\right)\)
\(\Rightarrow V_{O2}=0,75.22,4=16,8\left(l\right)\)
b. \(n_P=\frac{3,1}{31}=0,1\left(mol\right)\)
\(4P+5O_2\rightarrow2P_2O_5\)
\(n_{O2}=\frac{5}{4}_P=\frac{5}{4}.0,1=0,125\left(mol\right)\)
\(\Rightarrow V_{O2}=0,125.22,4=2,8\left(l\right)\)
\(S+O_2\rightarrow SO_2\)
\(n_S=n_{O2}=\frac{6,4}{32}=0,2\left(mol\right)\)
\(\Rightarrow V_{O2}=0,2.22,4=4,48\left(l\right)\)
\(C+O_2\rightarrow CO_2\)
\(n_C=n_{O2}=\frac{3,6}{12}=0,3\left(mol\right)\)
\(\Rightarrow V_{O2}=0,3.22,4=6,72\left(l\right)\)
c.
\(CH_4+2O_2\rightarrow CO_2+2H_2O\)
\(n_{CH4}=\frac{1,6}{16}=0,1\left(mol\right)\)
\(n_{O2}=2n_{CH4}=0,2\left(mol\right)\)
\(\Rightarrow V_{O2}=0,2.33,4=4,48\left(l\right)\)
\(2CO+O_2\rightarrow2CO_2\)
\(n_{CO}=\frac{2,8}{28}=0,1\left(mol\right)\)
\(\Rightarrow V_{O2}=0,1.22,4=2,24\left(l\right)\)
\(13O_2+2C_4H_{10}\rightarrow10H_2O+8CO_2\)
\(n_{C4H10}=\frac{0,58}{58}=0,01\left(mol\right)\)
\(n_{O2}=\frac{2}{13}n_{C4H10}=\frac{2}{13}.0,01=0,0015\left(mol\right)\)
\(\Rightarrow n_{O2}=0,0015.22,4=0,034\left(l\right)\)
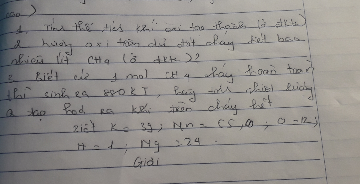

2KMnO4->K2MnO4+MnO2+O2
a)nMnO2=\(\frac{13,05}{87}=0,15\left(mol\right)\)
nO2=nMnO2=0,15(mol)
VO2=0,15.22,4=3,36l
b)nKMnO4=\(\frac{m_{KMnO_4}}{MKMnO_4}=\frac{63,2}{158}=0,4\left(mol\right)\)
nO2=\(\frac{1}{2}.n_{KMnO_4}=\frac{1}{2}.0,4=0,2\left(mol\right)\)
VO2=0,2.22,4=4,48(l)