Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{KOH}=0,03mol\)
\(n_{HCl}=0,027mol\)
\(OH^-+H^+\rightarrow H_2O\)
bđ 0,3 0,027 0
pư 0,027 0,027 0,027
kt 0,273 0 0,027
\(\left[OH\right]^-_{dư}=\dfrac{0.273}{0,15+0,15}=0,91\)
\(\Rightarrow pH_A=-log\left(\dfrac{10^{-14}}{0,91}\right)=13,96\)

a, \(n_{NaOH}=0,15.0,2=0,03\left(mol\right)=n_{Na^+}=n_{OH^-}\)
\(n_{KOH}=0,15.0,2=0,03\left(mol\right)=n_{K^+}=n_{OH^-}\)
⇒ ΣnOH- = 0,03 + 0,03 = 0,06 (mol)
\(n_{HCl}=0,25.0,4=0,1\left(mol\right)=n_{H^+}=n_{Cl^-}\)
\(H^++OH^-\rightarrow H_2O\)
0,06____0,06 (mol) ⇒ nH+ dư = 0,1 - 0,06 = 0,04 (mol)
\(\left[Na^+\right]=\left[K^+\right]=\dfrac{0,03}{0,15+0,25}=0,075\left(M\right)\)
\(\left[H^+\right]=\dfrac{0,04}{0,15+0,25}=0,1\left(M\right)\)
\(\left[Cl^-\right]=\dfrac{0,1}{0,15+0,25}=0,25\left(M\right)\)
b, pH = -log[H+] = 1

a, \(n_{HCl}=0,1.0,2=0,02\left(mol\right)=n_{H^+}=n_{Cl^-}\)
\(n_{H_2SO_4}=0,1.0,2=0,02\left(mol\right)=n_{SO_4^{2-}}\) \(\Rightarrow n_{H^+}=2n_{H_2SO_4}=0,04\left(mol\right)\)
\(n_{NaOH}=0,3.0,4=0,12\left(mol\right)=n_{Na^+}=n_{OH^-}\)
\(\Rightarrow\sum n_{H^+}=0,02+0,04=0,06\left(mol\right)\)
\(H^++OH^-\rightarrow H_2O\)
0,06__0,06 (mol)
⇒ nOH- dư = 0,12 - 0,06 = 0,06 (mol)
\(\Rightarrow\left\{{}\begin{matrix}\left[Cl^-\right]=\dfrac{0,02}{0,1+0,3}=0,05\left(M\right)\\\left[SO_4^{2-}\right]=\dfrac{0,02}{0,1+0,3}=0,05\left(M\right)\\\left[Na^+\right]=\dfrac{0,12}{0,1+0,3}=0,3\left(M\right)\\\left[OH^-\right]=\dfrac{0,06}{0,1+0,3}=0,15\left(M\right)\end{matrix}\right.\)
b, pH = 14 - (-log[OH-]) ≃ 13,176

\(n_{NaOH}=0,006\left(mol\right)\\ \Rightarrow n_{Na^+}=0,006\left(mol\right);n_{OH^-}=0,006\left(mol\right)\\ n_{H_2SO_4}=0,005\left(mol\right)\\ \Rightarrow n_{H^+}=0,01\left(mol\right);n_{SO_4^{2-}}=0,005\left(mol\right)\\ H^++OH^-\rightarrow H_2O\\ LTL:\dfrac{0,01}{1}>\dfrac{0,006}{1}\Rightarrow H^+dư\\ \left[H^+_{dư}\right]=\dfrac{0,01-0,006}{0,1}=0,04M\\ \left[Na^+\right]=\dfrac{0,006}{0,1}=0,06M\\ \left[SO_4^{2-}\right]=\dfrac{0,005}{0,1}=0,05M\)

Chọn D
Trộn 3 dung dịch với thể tích bằng nhau thu được 150 ml dung dịch X → mỗi dung dịch lấy 50ml.
→ n H + = 0,05.0,2 + 0,05.2.0,1 + 0,05.0,08 = 0,024 mol.
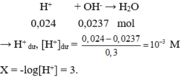
\(n_{NaOH}=0.2\cdot0.05=0.01\left(mol\right)\)
\(C_{M_{NaOH\left(ls\right)}}=\dfrac{0.01}{0.05+0.15}=0.05\left(M\right)\)
\(pH=14+log\left(0.05\right)=12.69\)