Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

Gọi: \(\left\{{}\begin{matrix}n_{Mg}=x\left(mol\right)\\n_{Fe}=y\left(mol\right)\end{matrix}\right.\) ⇒ 24x + 56y = 16 (1)
Có: m dd tăng = mMg + mFe - mH2
⇒ mH2 = 16 - 15,2 = 0,8 (g) \(\Rightarrow n_{H_2}=\dfrac{0,8}{2}=0,4\left(mol\right)\)
BT e, có: 2nMg + 2nFe = 2nH2 ⇒ x + y = 0,4 (2)
Từ (1) và (2) ⇒ x = y = 0,2 (mol)
BTNT H, có: nHCl = 2nH2 = 0,8 (mol)
\(\Rightarrow m_{ddHCl}=\dfrac{0,8.36,5}{20\%}=146\left(g\right)\)
⇒ m dd sau pư = 146 + 15,2 = 161,2 (g)
BTNT Mg, có: nMgCl2 = nMg = 0,2 (mol)
\(\Rightarrow C\%_{MgCl_2}=\dfrac{0,2.95}{161,2}.100\%\approx11,79\%\)

\(n_{H_2}=\dfrac{11,2}{22,4}=0,5\left(mol\right)\)
Gọi \(\left\{{}\begin{matrix}m_{Mg}=a\\n_{Fe}=b\end{matrix}\right.\) => 24a + 56b = 20
PTHH: Mg + 2HCl --> MgCl2 + H2
______a------------------>a------>a__________(mol)
Fe + 2HCl --> FeCl2 + H2
_b----------------->b----->b__________________(mol)
=> a+b = 0,5
=> \(\left\{{}\begin{matrix}a=0,25\\b=0,25\end{matrix}\right.\) => \(\left\{{}\begin{matrix}m_{MgCl_2}=0,25.95=23,75\left(g\right)\\m_{FeCl_2}=0,25.127=31,75\left(g\right)\end{matrix}\right.\) => m muối = 55,5(g)

\(Đặt:\)
\(\left\{{}\begin{matrix}n_{Mg}=x\left(mol\right)\\\\n_{Fe}=y\left(mol\right)\end{matrix}\right.\)
\(n_{H_2}=\dfrac{6.72}{22.4}=0.3\left(mol\right)\)
\(Mg+2HCl\rightarrow MgCl_2+H_2\\ Fe+2HCl\rightarrow FeCl_2+H_2\)
\(m_{hh}=24x+56y=13.6\left(g\right)\\ n_{H_2}=x+y=0.3\left(mol\right)\)
\(\Rightarrow\left\{{}\begin{matrix}x=0.1\\y=0.2\end{matrix}\right.\)
\(\%Mg=\dfrac{0.1\cdot24}{13.6}\cdot100\%=17.64\%\\ \%Fe=100-17.64=82.36\%\)
\(n_{HCl}=2n_{H_2}=2\cdot0.3=0.6\left(mol\right)\)
\(V_{HCl}=\dfrac{0.6}{2}=0.3\left(l\right)\)
\(m_Y=m_{MgCl_2}+m_{FeCl_2}=0.1\cdot95+0.2\cdot127=34.9\left(g\right)\)

\(Đặt:n_{Al}=a\left(mol\right),n_{Fe}=b\left(mol\right)\)
\(m_{hh}=27a+56b=8.3\left(g\right)\left(1\right)\)
\(n_{H_2}=\dfrac{5.6}{22.4}=0.25\left(mol\right)\)
\(2Al+6HCl\rightarrow2AlCl_3+3H_2\)
\(Fe+2HCl\rightarrow FeCl_2+H_2\)
\(Tathấy:\)
\(n_{HCl}=2n_{H_2}=2\cdot0.25=0.5\left(mol\right)\)
\(V_{ddHCl}=\dfrac{0.5}{0.2}=2.5\left(l\right)\)
\(n_{H_2}=1.5a+b=0.25\left(mol\right)\left(2\right)\)
\(\left(1\right),\left(2\right):a=b=0.1\)
\(C_{M_{AlCl_3}}=\dfrac{0.1}{2.5}=0.04\left(M\right)\)
\(C_{M_{FeCl_2}}=\dfrac{0.1}{2.5}=0.04\left(M\right)\)
Chúc em học tốt !!!
a, Ta có: \(n_{H_2}=\dfrac{5,6}{22,4}=0,25\left(mol\right)\)
BTNT H, có: \(n_{HCl}=2n_{H_2}=0,5\left(mol\right)\)
\(\Rightarrow V_{HCl}=\dfrac{0,5}{0,2}=2,5\left(l\right)\)
b, Giả sử: \(\left\{{}\begin{matrix}n_{Al}=x\left(mol\right)\\n_{Fe}=y\left(mol\right)\end{matrix}\right.\)
⇒ 27x + 56y = 8,3 (1)
Các quá trình:
\(Al^0\rightarrow Al^{+3}+3e\)
x___________ 3x (mol)
\(Fe^0\rightarrow Fe^{+2}+2e\)
y____________2y (mol)
\(2H^++2e\rightarrow H_2^0\)
______0,5__0,25 (mol)
Theo ĐLBT mol e, có: 3x + 2y = 0,5 (2)
Từ (1) và (2) ⇒ x = y = 0,1 (mol)
BTNT Al và Fe, có: \(\left\{{}\begin{matrix}n_{AlCl_3}=n_{Al}=0,1\left(mol\right)\\n_{FeCl_3}=n_{Fe}=0,1\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow C_{M_{AlCl_3}}=C_{M_{FeCl_3}}=\dfrac{0,1}{2,5}=0,04M\)
Bạn tham khảo nhé!

a) PTHH: \(Fe+2HCl\rightarrow FeCl_2+H_2\uparrow\) (1)
\(Mg+2HCl\rightarrow MgCl_2+H_2\uparrow\) (2)
b) Dựa vào đề, ta thấy chắc chắn HCl dư
Ta có: \(\Sigma n_{H_2}=\dfrac{4,48}{22,4}=0,2\left(mol\right)\)
Gọi số mol của Fe là \(a\) \(\Rightarrow n_{H_2\left(1\right)}=a\)
Gọi số mol của Mg là \(b\) \(\Rightarrow n_{H_2\left(2\right)}=b\)
Ta lập được hệ phương trình:
\(\left\{{}\begin{matrix}56a+24b=8\\a+b=0,2\end{matrix}\right.\) \(\Leftrightarrow\left\{{}\begin{matrix}a=0,1\\b=0,1\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}m_{Fe}=56\cdot0,1=5,6\left(g\right)\\m_{Mg}=24\cdot0,1=2,4\left(g\right)\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}\%m_{Fe}=\dfrac{5,6}{8}\cdot100\%=70\%\\\%m_{Mg}=30\%\end{matrix}\right.\)
c) Theo các PTHH: \(n_{FeCl_2}=n_{MgCl_2}=n_{Fe}=n_{Mg}=0,1mol\)
\(\Rightarrow\left\{{}\begin{matrix}m_{FeCl_2}=0,1\cdot127=12,7\left(g\right)\\m_{MgCl_2}=0,1\cdot95=9,5\left(g\right)\end{matrix}\right.\) \(\Rightarrow m_{muối}=22,2\left(g\right)\)
d) Ta có: \(\Sigma n_{HCl}=\dfrac{500\cdot16\%}{36,5}=\dfrac{160}{73}\left(mol\right)\)
\(\Rightarrow n_{HCl\left(dư\right)}=\dfrac{654}{365}\left(mol\right)\) \(\Rightarrow m_{HCl\left(dư\right)}=\dfrac{654}{365}\cdot36,5=65,4\left(g\right)\)
Mặt khác: \(m_{dd}=m_{hh}+m_{ddHCl}-m_{H_2}=507,6\left(g\right)\)
\(\Rightarrow\left\{{}\begin{matrix}C\%_{FeCl_2}=\dfrac{12,7}{507,6}\cdot100\%\approx2,5\%\\C\%_{MgCl_2}=\dfrac{9,5}{507,6}\cdot100\%\approx1,87\%\\C\%_{HCl\left(dư\right)}=\dfrac{65,4}{507,6}\cdot100\%\approx12,88\%\end{matrix}\right.\)
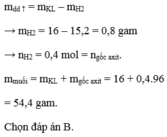
m tăng= m kim loại - mH2
\(m_{H2}=0,8\left(g\right)\)
\(\Rightarrow n_{H2}=0,4\left(mol\right)\)
Gọi a là mol Mg, b là mol Fe
\(\Rightarrow24a+56b=12,8\left(1\right)\)
Bảo toàn e: \(2a+2b=0,4.2=0,8\left(2\right)\)
\(\left(1\right)+\left(2\right)\Rightarrow\left\{{}\begin{matrix}a=0,3\left(mol\right)\\b=0,1\left(mol\right)\end{matrix}\right.\)