Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(a)2Na+2HCl\xrightarrow[]{}2NaCl+H_2\\ 2K+2HCl\xrightarrow[]{}2KCl+H_2 \\ b)n_{H_2}=\dfrac{2,24}{22,4}=0,1mol\\ n_{Na}=a,n_K=b\\ \Rightarrow\left\{{}\begin{matrix}23a+39b=6,2\\\dfrac{1}{2}a+\dfrac{1}{2}b=0,1\end{matrix}\right.\\ \Rightarrow a=b=0,1mol\\ \%_{Na}=\dfrac{0,1.23}{6,2}\cdot100=37,1\%\\ \%_K=100-37,1=62,9\%\)

a) PTHH: \(Fe+2HCl\rightarrow FeCl_2+H_2\uparrow\)
a_____2a______a_____a (mol)
\(2Al+6HCl\rightarrow2AlCl_3+3H_2\uparrow\)
b_____3b_______b_____\(\dfrac{3}{2}\)b (mol)
Ta lập HPT: \(\left\{{}\begin{matrix}56a+27b=36,1\\a+\dfrac{3}{2}b=\dfrac{21,28}{22,4}=0,95\end{matrix}\right.\) \(\Leftrightarrow\left\{{}\begin{matrix}a=0,5\\b=0,3\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}m_{Fe}=0,5\cdot56=28\left(g\right)\\m_{Al}=8,1\left(g\right)\end{matrix}\right.\)
b+c) Theo các PTHH: \(\left\{{}\begin{matrix}n_{HCl}=2a+3b=1,9\left(mol\right)\\n_{FeCl_2}=0,5\left(mol\right)\\n_{AlCl_3}=0,3\left(mol\right)\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}C_{M_{HCl}}=\dfrac{1,9}{0,2}=9,5\left(M\right)\\C_{M_{FeCl_2}}=\dfrac{0,5}{0,2}=2,5\left(M\right)\\C_{M_{AlCl_3}}=\dfrac{0,3}{0,2}=1,5\left(M\right)\end{matrix}\right.\)

2Al + 2NaOH + 2H2O \(\rightarrow\) 2NaAlO2 + 3H2
CR X là Fe
Fe + 2HCl \(\rightarrow\) FeCl2 + H2
0,1 <----------------------- 0,1
%mFe = 50,91%
%mAl = 49,09%
cho mình bk lí do vì sao mà Al lại + với NaOH và H2O đc ko H2O ở đâu ra vậy bạn

a, \(Mg+H_2SO_4\rightarrow MgSO_4+H_2\)
\(MgO+H_2SO_4\rightarrow MgSO_4+H_2O\)
b, \(n_{H_2}=\dfrac{2,24}{22,4}=0,1\left(mol\right)\)
Theo PT: \(n_{Mg}=n_{H_2}=0,1\left(mol\right)\Rightarrow m_{Mg}=0,1.24=2,4\left(g\right)\)
\(\Rightarrow m_{MgO}=4,4-2,4=2\left(g\right)\)
c, \(n_{MgO}=\dfrac{2}{40}=0,05\left(mol\right)\)
Theo PT: \(n_{H_2SO_4}=n_{Mg}+n_{MgO}=0,15\left(mol\right)\)
\(\Rightarrow m_{H_2SO_4}=0,15.98=14,7\left(g\right)\)
\(\Rightarrow m_{ddH_2SO_4}=\dfrac{14,7}{19,6\%}=75\left(g\right)\)

\(Mg+H_2SO_4\rightarrow MgSO_4+H_2\)
tl1..........1................1.............1(mol)
br x.......x................x.............x(mol)
\(Cu+H_2SO_4\rightarrow CuSO_4+H_2\)
tl1............1...............1...........1(mol)
Br y...........y...............y...........y(mol)
\(n_{H_2}=\dfrac{1,12}{22,4}=0,05\left(mol\right)\)
Taco hệ pt
\(\left\{{}\begin{matrix}x+y=0,05\\24x+64y=5\end{matrix}\right.\Rightarrow\left\{{}\begin{matrix}x=0,045\\y=0,095\end{matrix}\right.\)
\(\Rightarrow\%m_{Mg}=0,045.24:5.100\%=21,6\%\)
\(\Rightarrow\%m_{Cu}=100\%-21,6\%=78,4\%\)

Đáp án B
Cho Al và Ag phản ứng với H 2 S O 4 loãng, dư chỉ có Al phản ứng.
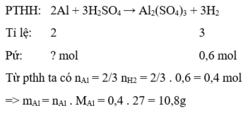
% m A l = 10,8 12 .100 % = 90 % .
% m A g = 100 % - 90 % = 10 %
$2Al + 3H_2SO_4 \to Al_2(SO_4)_3 + 3H_2$
$n_{H_2} = \dfrac{2,24}{22,4} = 0,1(mol)$
Theo PTHH :
$n_{Al} = \dfrac{2}{3}n_{H_2} = \dfrac{0,2}{3}(mol)$
$\%m_{Al} = \dfrac{ \dfrac{0,2}{3}.27}{16}.100\% = 11,25\%$
$\%m_{Cu} = 100\% - 11,25\% = 88,75\%$