Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{Fe_2O_3}=\dfrac{16}{160}=0,1\left(mol\right)\)
PTHH: Fe2O3 + 3H2 --to--> 2Fe + 3H2O
0,1 0,3 0,2
\(V_{H_2}=0,3.22,4=6,72\left(l\right)\)
\(n_{O_2}=\dfrac{2,24}{22,4}=0,1\left(mol\right)\)
PTHH: 3Fe + 2O2 --to--> Fe3O4
LTL: \(\dfrac{0,2}{3}>\dfrac{0,1}{2}\rightarrow\) Fe dư
Theo pthh: \(n_{Fe\left(pư\right)}=\dfrac{3}{2}n_{O_2}=\dfrac{3}{2}.0,1=0,15\left(mol\right)\)
\(\rightarrow m_{Fe\left(dư\right)}=\left(0,2-0,15\right).56=2,8\left(g\right)\)
a.\(n_{Fe_2O_3}=\dfrac{16}{160}=0,1mol\)
\(Fe_2O_3+3H_2\rightarrow\left(t^o\right)2Fe+3H_2O\)
0,1 0,3 0,2 ( mol )
\(V_{H_2}=0,3.22,4=6,72l\)
b.\(n_{O_2}=\dfrac{2,24}{22,4}=0,1mol\)
\(3Fe+2O_2\rightarrow\left(t^o\right)Fe_3O_4\)
\(\dfrac{0,2}{3}\) > \(\dfrac{0,1}{2}\) ( mol )
0,15 0,1 ( mol )
Chất dư là Fe
\(m_{Fe\left(dư\right)}=\left(0,2-0,15\right).56=2,8g\)

a)\(n_{Fe_3O_4}=\dfrac{69,6}{232}=0,3\left(m\right)\)
\(PTHH:3Fe+2O_2\xrightarrow[]{}Fe_3O_4\)
tỉ lệ : 3mol 2mol 1mol
số mol : 0,9 0,6 0,3
\(m_{Fe}=0,9.56=50,4\left(g\right)\)
\(V_{O_2}=0,6.22,4=13,44\left(l\right)\)
b)\(PTHH:2KClO_3\underrightarrow{t^o}2KCl+3O_2\)
tỉ lệ : 2mol 2mol 3mol
số mol : 0,4 0,4 0,6
\(m_{KClO_3}=122,5.0,4=49\left(g\right)\)

\(PTHH:Fe_3O_4+4H_2\rightarrow^{t^o}3Fe+4H_2O\\ n_{Fe}=\dfrac{30,24}{56}=0,54\left(mol\right)\\ \Rightarrow n_{Fe_3O_4}=\dfrac{1}{3}n_{Fe}=0,18\left(mol\right)\\ \Rightarrow m_{Fe_3O_4}=0,18\cdot232=41,76\left(g\right)\)

a) PTHH:
\(4CO+Fe_3O_4\rightarrow3Fe+4CO_2\left(1\right)\)
\(3H_2+Fe_2O_3\rightarrow2Fe+3H_2O\left(2\right)\)
b) Theo PTPƯ trên ta có:
Muốn khử 1 mol \(Fe_3O_4\) cần 4 mol CO
Muốn khử 0,2 mol \(Fe_3O_4\) cần x mol CO
\(\Rightarrow x=0,2.4=0,8\left(mol\right)\)
\(V_{CO}=0,8.22,4=17,92\left(l\right)\)
Muốn khử 1 mol \(Fe_2O_3\) cần 3 mol H2
Muốn khử 0,2 mol \(Fe_2O_3\) cần y mol H2
\(\Rightarrow y=0,2.3=0,6\left(mol\right)\)
\(V_{H_2}=0,6.22,4=13,44\left(l\right)\)
c) (1): \(n_{Fe}=3n_{Fe_3O_4}=3.0,2=0,6\left(mol\right)\)
\(m_{Fe}=0,6.56=33,6\left(g\right)\)
(2): \(n_{Fe}=2n_{Fe_2O_3}=2.0,2=0,4\left(mol\right)\)
\(m_{Fe}=0,4.56=22,4\left(g\right)\)

a, \(Fe_2O_3+3H_2\underrightarrow{t^o}2Fe+3H_2O\)
b, \(n_{Fe}=\dfrac{22,4}{56}=0,4\left(mol\right)\)
Theo PT: \(n_{Fe_2O_3}=\dfrac{1}{2}n_{Fe}=0,2\left(mol\right)\Rightarrow m_{Fe_2O_3}=0,2.160=32\left(g\right)\)
c, \(n_{H_2}=\dfrac{3}{2}n_{Fe}=0,6\left(mol\right)\Rightarrow V_{H_2}=0,6.22,4=13,44\left(l\right)\)
d, \(2H_2+O_2\underrightarrow{t^o}2H_2O\)
Theo PT: \(n_{O_2}=\dfrac{1}{2}n_{H_2}=0,3\left(mol\right)\Rightarrow V_{O_2}=0,3.22,4=6,72\left(l\right)\)
\(\Rightarrow V_{kk}=\dfrac{V_{O_2}}{20\%}=33,6\left(l\right)\)
a)
$Fe_2O_3 + 3H_2 \xrightarrow{t^o} 2Fe + 3H_2O$
b) $n_{Fe} = \dfrac{22,4}{56} = 0,4(mol)$
Theo PTHH : $n_{Fe_2O_3} = \dfrac{1}{2}n_{Fe} = 0,2(mol)$
$m_{Fe_2O_3} = 0,2.160 = 32(gam)$
c) $n_{H_2} = \dfrac{3}{2}n_{Fe} = 0,6(mol)$
$V_{H_2} = 0,6.22,4 = 13,44(lít)$
d) $2H_2 + O_2 \xrightarrow{t^o} 2H_2O$
$V_{O_2} = \dfrac{1}{2}V_{H_2} = 6,72(lít)$
$V_{kk} = 6,72 : 20\% = 33,6(lít)$

a, PT: \(Zn+2HCl\rightarrow ZnCl_2+H_2\)
b, Ta có: \(n_{Zn}=\dfrac{6,5}{65}=0,1\left(mol\right)\)
Theo PT: \(n_{H_2}=n_{Zn}=0,1\left(mol\right)\Rightarrow V_{H_2}=0,1.22,4=2,24\left(l\right)\)
c, PT: \(Fe_2O_3+3H_2\underrightarrow{t^o}2Fe+3H_2O\)
Theo PT: \(n_{Fe}=\dfrac{2}{3}n_{H_2}=\dfrac{1}{15}\left(mol\right)\Rightarrow m_{Fe}=\dfrac{1}{15}.56=\dfrac{56}{15}\left(g\right)\)
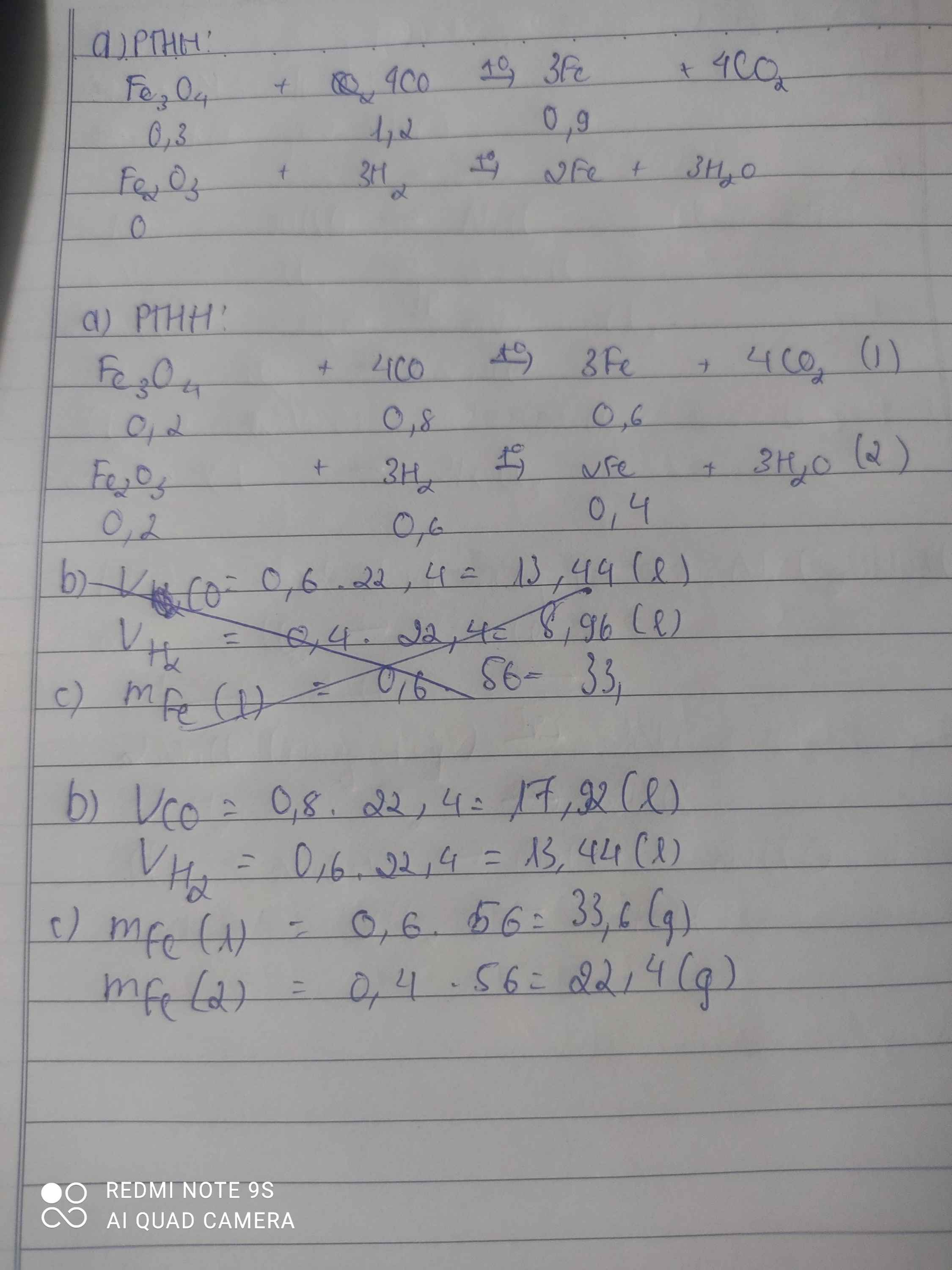
\(Fe_2O_3+3H_2\underrightarrow{t^o}2Fe+3H_2O\)
0,05 0,1 0,15
\(n_{Fe}=\dfrac{5,6}{56}=0,1\left(mol\right)\)
\(a,m_{Fe_2O_3}=0,05.8\left(g\right)\)
\(b,H_2SO_4+Fe\rightarrow FeSO_4+H_2\uparrow\)
0,15 0,15 0,15
\(m_{ddH_2SO_4}=\dfrac{0,15.98.100}{50}=29,4\left(g\right)\)
\(m_{Fe}=\dfrac{0,15.56.100}{50}=16,8\left(g\right)\)