
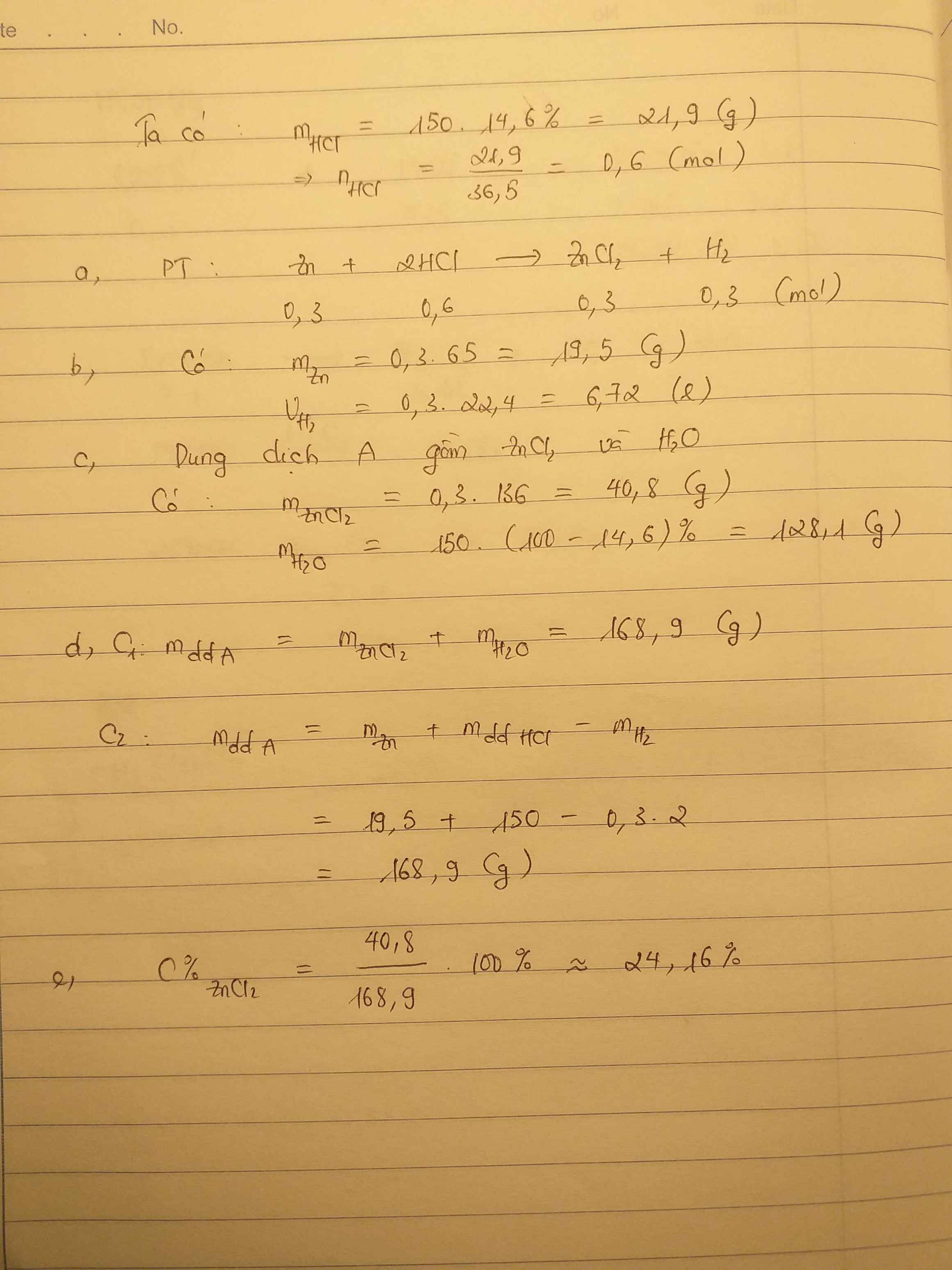
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

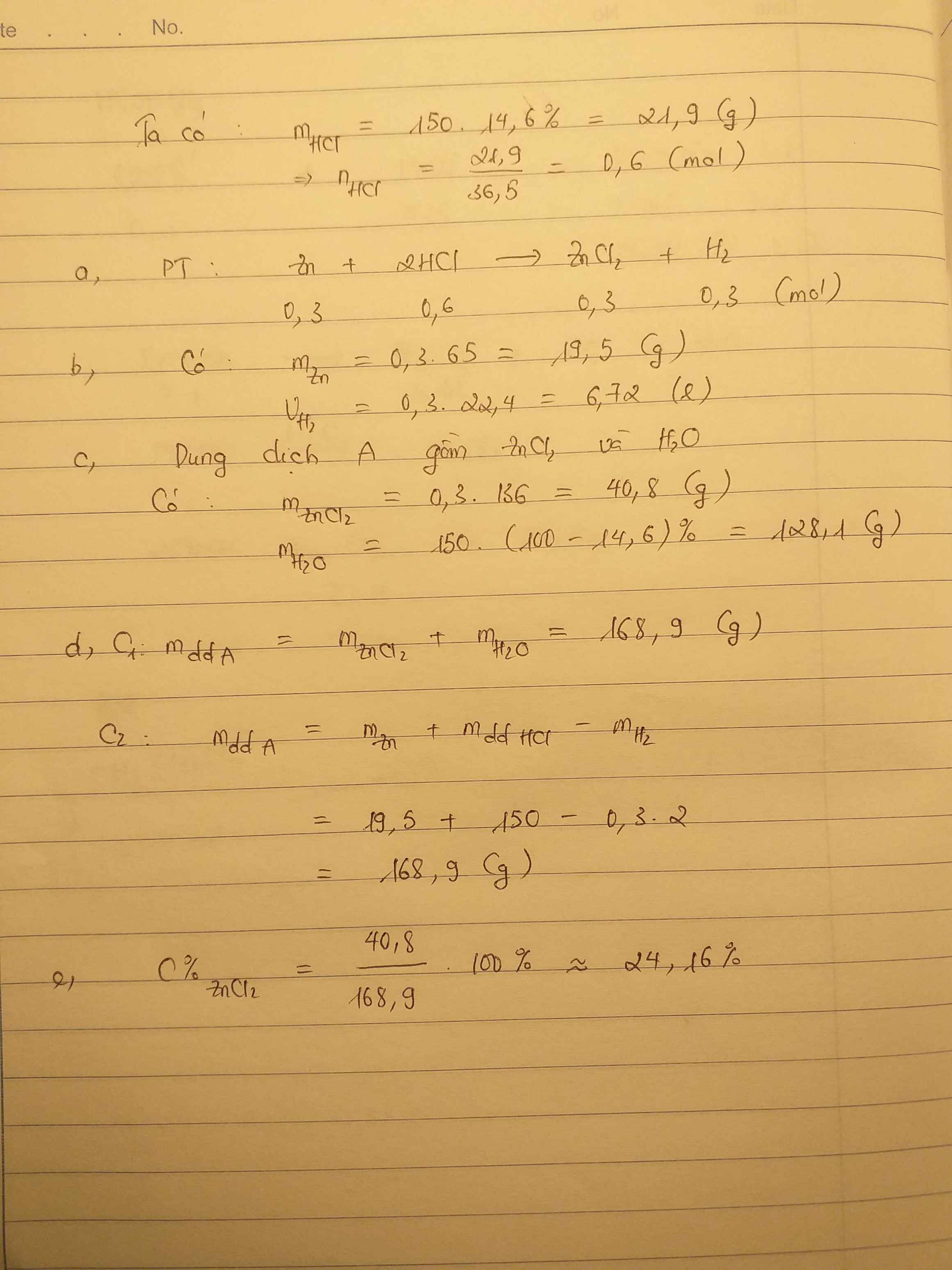

\(n_{H_2}=\dfrac{3,36}{22,4}=0,15\left(mol\right)\\ PTHH:Zn+2HCl\rightarrow ZnCl_2+H_2\\ n_{ZnCl_2}=n_{Zn}=n_{H_2}=0,15\left(mol\right);n_{HCl}=2.0,15=0,3\left(mol\right)\\ a,m=m_{Zn}=0,15.65=9,75\left(g\right)\\ b,C_{MddHCl}=\dfrac{0,3}{0,15}=0,2\left(l\right)\\ c,m_{ZnCl_2}=0,15.136=20,4\left(g\right)\)

a)
\(n_{Zn}=\dfrac{13}{65}=0,2\left(mol\right)\)
PTHH: Zn + 2HCl --> ZnCl2 + H2
0,2-->0,4----->0,2--->0,2
=> VH2 = 0,2.22,4 = 4,48 (l)
b) mHCl = 0,4.36,5 = 14,6 (g)
=> \(m_{dd.HCl}=\dfrac{14,6.100}{7,3}=200\left(g\right)\)
c)
mdd sau pư = 13 + 200 - 0,2.2 = 212,6 (g)
mZnCl2 = 0,2.136 = 27,2 (g)
=> \(C\%=\dfrac{27,2}{212,6}.100\%=12,8\%\)

a, \(Zn+2HCl\rightarrow ZnCl_2+H_2\)
b, \(n_{Zn}=\dfrac{13}{65}=0,2\left(mol\right)\)
Theo PT: \(n_{H_2}=n_{Zn}=0,2\left(mol\right)\Rightarrow V_{H_2}=0,2.22,4=4,48\left(l\right)\)
c, \(n_{ZnCl_2}=n_{Zn}=0,2\left(mol\right)\Rightarrow m_{ZnCl_2}=0,2.136=27,2\left(g\right)\)
d, \(n_{HCl}=2n_{Zn}=0,4\left(mol\right)\Rightarrow m_{HCl}=0,4.36,5=14,6\left(g\right)\)
\(\Rightarrow m_{ddHCl}=\dfrac{14,6}{7,3\%}=200\left(g\right)\)
⇒ m dd sau pư = 13 + 200 - 0,2.2 = 212,6 (g)
\(\Rightarrow C\%_{ZnCl_2}=\dfrac{27,2}{212,6}.100\%\approx12,79\%\)

a,Fe + 2HCl → FeCl + H2 (1)
FeO + 2HCl → FeCl + H2O (2)
nH2 = 3,36/ 22,4 = 0,15 ( mol)
Theo (1) nH2 = nFe = 0,15 ( mol)
mFe = 0,15 x 56 = 8.4 (g)
m FeO = 12 - 8,4 = 3,6 (g)
a, \(n_{H_2}=\frac{3,36}{22,4}=0,15\left(mol\right)\)
\(Fe+2HCl->FeCl_2+H_2\left(1\right)\)
\(FeO+2HCl->FeCl_2+H_2O\left(2\right)\)
theo (1) \(n_{Fe}=n_{H_2}=0,15\left(mol\right)\)
=> \(m_{Fe}=0,15.56=8,4\left(g\right)\)
=> \(m_{FeO}=12-8,4=3,6\left(g\right)\)

ta thấy : nFe =nH2 = 0,15
=> mFe =0,15 x 56 = 8,4g
%Fe=8,4/12 x 100 = 70%
=>%FeO = 100 - 70 = 30%
b) BTKLra mdd tìm mct of HCl
c) tìm mdd sau pứ -mH2 nha bạn

a, \(n_{Zn}=\dfrac{6,5}{65}=0,1\left(mol\right)\)
PT: \(Zn+2HCl\rightarrow ZnCl_2+H_2\)
____0,1_____0,2______0,1_____0,1 (mol)
\(V_{H_2}=0,1.24,79=2,479\left(l\right)\)
\(m_{ZnCl_2}=0,1.136=13,6\left(g\right)\)
b, \(C_{M_{HCl}}=\dfrac{0,2}{0,1}=1\left(M\right)\)
c, \(n_{CuO}=\dfrac{12}{80}=0,15\left(mol\right)\)
PT: \(CuO+H_2\underrightarrow{t^o}Cu+H_2O\)
Xét tỉ lệ: \(\dfrac{0,15}{1}>\dfrac{0,1}{1}\), ta được CuO dư.
Theo PT: \(n_{CuO\left(pư\right)}=n_{Cu}=n_{H_2}=0,1\left(mol\right)\Rightarrow n_{CuO\left(dư\right)}=0,05\left(mol\right)\)
⇒ m chất rắn = mCuO (dư) + mCu = 0,05.80 + 0,1.64 = 10,4 (g)

\(n_{Zn}=\dfrac{8,125}{65}=0,125\left(mol\right)\\ m_{HCl}=\dfrac{100.18,25}{100}=18,25\left(g\right)\\
n_{HCl}=\dfrac{18,25}{36,5}=0,5\\ pthh:Zn+2HCl\rightarrow ZnCl_2+H_2\uparrow\)
0,125 0,125 (mol )
\(\Rightarrow V_{H_2}=0,125.22,4=2,8\left(l\right)\\
\)
\(C\%=\dfrac{8,125}{8,125+18,25}.100\%=30,8\%\)
Bài 18:
Ta có: \(n_{Zn}=\dfrac{8,125}{65}=0,125\left(mol\right)\)
\(m_{HCl}=100.18,25\%=18,25\left(g\right)\Rightarrow n_{HCl}=\dfrac{18,25}{36,5}=0,5\left(mol\right)\)
a, PT: \(Zn+2HCl\rightarrow ZnCl_2+H_2\)
b, Xét tỉ lệ: \(\dfrac{0,125}{1}< \dfrac{0,5}{2}\), ta được HCl dư.
Theo PT: \(n_{H_2}=n_{Zn}=0,125\left(mol\right)\)
\(\Rightarrow V_{H_2}=0,125.22,4=2,8\left(g\right)\)
\(m_{H_2}=0,125.2=0,25\left(g\right)\)
c, Theo PT: \(\left\{{}\begin{matrix}n_{ZnCl_2}=n_{Zn}=0,125\left(mol\right)\\n_{HCl\left(pư\right)}=2n_{Zn}=0,25\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow n_{HCl\left(dư\right)}=0,25\left(mol\right)\)
Có: m dd sau pư = 8,125 + 100 - 0,25 = 107,875 (g)
\(\Rightarrow\left\{{}\begin{matrix}C\%_{ZnCl_2}=\dfrac{0,125.136}{107,875}.100\%\approx15,76\%\\C\%_{HCl\left(dư\right)}=\dfrac{0,25.36,5}{107,875}.100\%\approx8,46\%\end{matrix}\right.\)
Bạn tham khảo nhé!