Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a, \(m_{HCl}=150.7,3\%=10,95\left(g\right)\Rightarrow n_{HCl}=\dfrac{10,95}{36,5}=0,3\left(mol\right)\)
PT: \(Mg+2HCl\rightarrow MgCl_2+H_2\)
Theo PT: \(n_{Mg}=n_{MgCl_2}=n_{H_2}=\dfrac{1}{2}n_{HCl}=0,15\left(mol\right)\)
\(m_{Mg}=0,15.24=3,6\left(g\right)\)
b, \(V_{H_2}=0,15.22,4=3,36\left(l\right)\)
c, Ta có: m dd sau pư = 3,6 + 150 - 0,15.2 = 153,3 (g)
\(\Rightarrow C\%_{MgCl_2}=\dfrac{0,15.95}{153,3}.100\%\approx9,3\%\)

\(n_{Zn}=\dfrac{16,25}{65}=0,25(mol)\\ \Rightarrow n_{H_2}=0,25(mol)\\ \Rightarrow V_{H_2}=0,25.24,79=6,1975(l)\\ \Rightarrow C\)

nZn=0,4mol
nH2SO4=0,5mol
PTHH: Zn+H2SO4=>ZnSO4+H2
0,4:0,5=> nH2SO4 dư theo nZn
p/ư: 0,4->0,4----->0m4---->0,4
=> VH2=0,4.22,4=8,96ml
b) mZnSO4 tạo thành : m=0,4.161=64,4g
c) ta có mđ H2SO4=1,12.500=560g
mddZnSO4=26+560-0,4.2=585,2g
=> C%(ZnSO4)=64,4:585,2.100=11%

25 độ , 1bar là điều kiện chuẩn
\(PTHH:Zn+H_2SO_4->ZnSO_4+H_2\)
0,25----------------------------->0,25 (mol)
\(n_{Zn}=\dfrac{m}{M}=\dfrac{16,25}{65}=0,25\left(mol\right)\)
\(V_{H_2\left(dkc\right)}=n\cdot24,79=0,25\cdot24,79=6,1975\left(l\right)\\ =>C\)

nH2SO4 = 49/98 = 0.5 (mol)
CMH2SO4 = 0.5/0.15 = 3.3 (M)
Zn + H2SO4 => ZnSO4 + H2
...........0.5.............0.5.........0.5
VH2 = 0.5 * 22.4 = 11.2 (l)
CMZnSO4 = 0.5 / 0.15 = 10/3 (M)
C%ZnSO4 = CM*M / 10D = 10/3 * 161 / 10 * 1.25 = 42.9 %

\(n_{Zn}=\dfrac{6,5}{65}=0,1\left(mol\right)\)
PTHH:
\(Zn+H_2SO_4\rightarrow ZnSO_4+H_2\)
0,1 0,1 0,1 0,1
\(m_{H_2SO_4}=0,1.98=9,8\left(g\right)\)
\(m_{ZnSO_4}=0,1.161=16,1\left(g\right)\)
\(V_{H_2}=0,1.24,79=2,479\left(l\right)\)

a,\(n_{Zn}=\dfrac{9,75}{65}=0,15\left(mol\right)\)
PTHH: Zn + H2SO4 → ZnSO4 + H2
Mol: 0,15 0,15 0,15
\(\Rightarrow C_{M_{ddH_2SO_4}}=\dfrac{0,15}{0,06}=2,5M\)
b, \(V_{H_2}=0,15.22,4=3,36\left(l\right)\)

a, Ta có:
nZn = 13/65= 0,2(mol)
PTHH: Zn + H2SO4 → ZnSO4 + H2
0,2-----------------------------------0,2
Theo PT : nZnSO4 = 0,2.1/1 = 0,2(mol)
mZnSO4 = 0,2. 161 = 32,2(g)
b, Ta có:
Theo PT : nH2 = 0,2.1/1 = 0,2(mol)
VH2(đktc) = 0,2 . 22,4 = 4,48(l)
CuO+H2-to>Cu+H2O
0,2-----0,2
=>m Cu=0,2.64=12,8g
Cậu ơi cho tớ hỏi ngu tý là cái mà "0,2---------0,2" là ntn vậy ạ :"))?
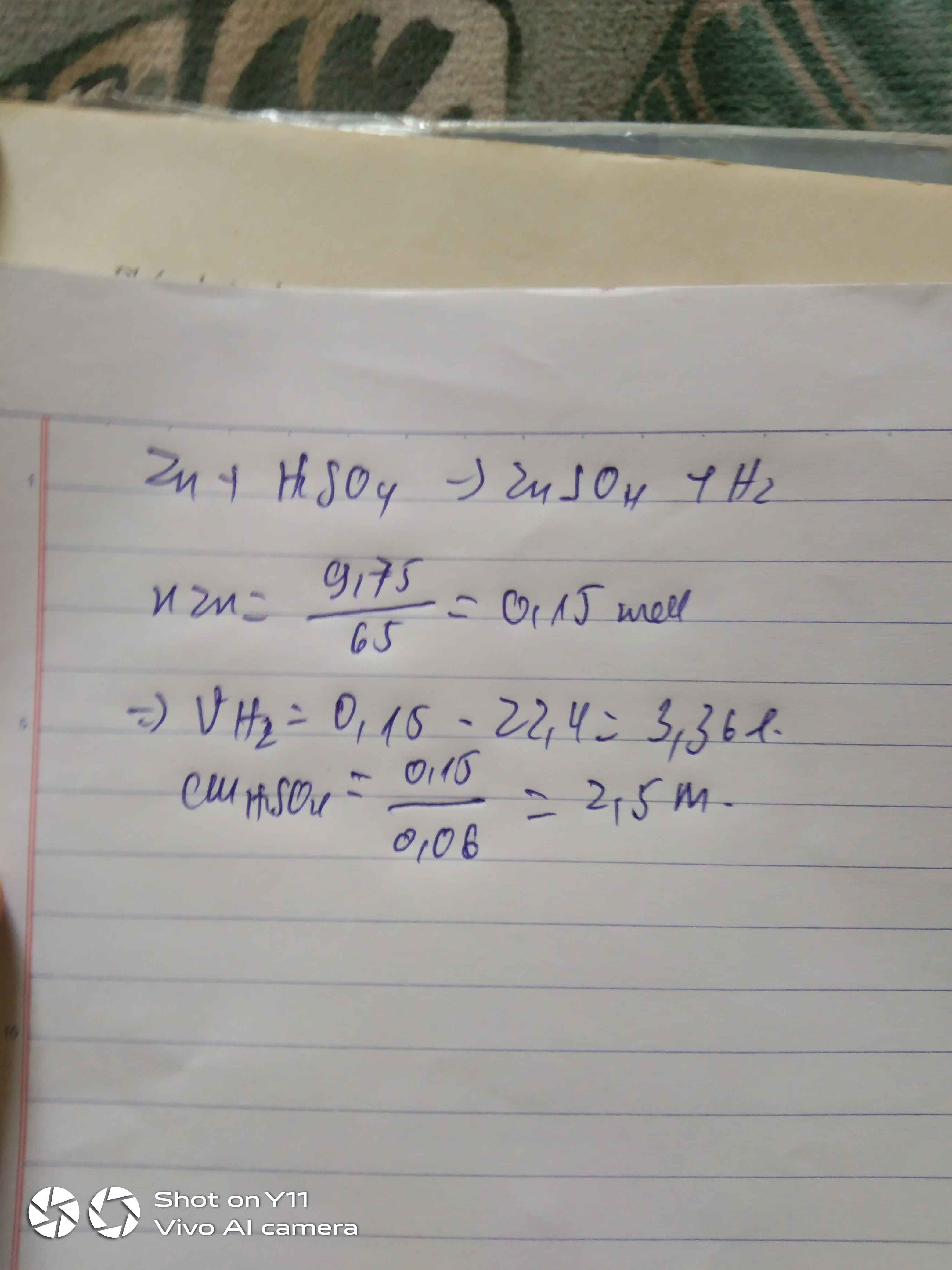
\(n_{Zn}=\dfrac{m}{M}=\dfrac{6,5}{65}=0,1\left(mol\right)\\ PTHH:Zn+H_2SO_4->ZnSO_4+H_2\)
tỉ lệ 1 ; 1 ; 1 ; 1
n(mol) 0,1-->0,1--------->0,1--------->0,1
\(V_{H_2\left(dktc\right)}=n\cdot22,4=0,1\cdot22,4=2,24\left(l\right)\\ m_{H_2SO_4}=n\cdot M=0,1\cdot\left(2+32+16\cdot4\right)=9,8\left(g\right)\\ m_{ZnSO_4}=n\cdot M=0,1\cdot\left(65+32+16\cdot4\right)=16,1\left(g\right)\)
a, \(n_{Zn}=\dfrac{6,5}{65}=0,1\left(mol\right)\)
PT: \(Zn+H_2SO_4\rightarrow ZnSO_4+H_2\)
____0,1____0,1________0,1____0,1 (mol)
\(\Rightarrow V_{H_2}=0,1.22,4=2,24\left(l\right)\)
b, \(m_{H_2SO_4}=0,1.98=9,8\left(g\right)\)
\(m_{ZnSO_4}=0,1.161=16,1\left(g\right)\)