Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(V_{C2H5OH}=12,5.92\%=11,5\left(ml\right)\)
\(\rightarrow m_{C2H5OH}=11,5.0,8=9,2\left(g\right)\rightarrow n_{C2H5OH}=\frac{9,2}{46}=0,2\left(mol\right)\)
\(C_2H_5OH+CuO\underrightarrow{^{to}}CH_3CHO+Cu+H_2O\)
\(\rightarrow n_{CH3CHO}=n_{C2H5OH}=0,2\left(mol\right)\)
\(CH_3CHO+2AgNO_3+2NH_3+H_2O\rightarrow CH_2COPNH_4+2Ag+2NH_4NO_3\)
\(\rightarrow n_{Ag}=2n_{CH3CHO}=0,4\left(mol\right)\)
\(\rightarrow m_{Ag}=0,4.108=43,2\left(g\right)\)

Ta có :
\(n_{C2H5OH}=0,58\left(mol\right)\)
\(C_2H_5OH+CuO\rightarrow CH_3CHO+Cu+H_2\)
0,58________________0,29_______________
\(CH_3CHO\rightarrow2Ag\)
0,29_______0,46
\(\rightarrow m_{Ag}=50,112\left(g\right)\)

\(n_{CH3OH}=\frac{17,92}{32}=0,56\left(mol\right)\)
\(CH_3OH+CuO\rightarrow HCHO+Cu+H_2O\)
\(\rightarrow n_{HCHO}=n_{CH3OH}=0,56\left(mol\right)\)\(HCHO+4AgNO_3+6NH_3+2H_2O\rightarrow4Ag+\left(NH_4\right)_2CO_3+4NH_4NO_3\)
\(\rightarrow n_{Ag}=4n_{HCHO}=0,56.4=2,24\left(mol\right)\)
\(\rightarrow m_{Ag}=2,24.108=241,92\left(g\right)\)

\(n_{C2H5OH}=0,123\left(mol\right)\)
\(C_2H_5OH+CuO\rightarrow Cu+CH_3CHO+H_2O\)
0,123_____________________0,123_______
\(CH_3CHO+2AgNO_3+3NH_3+H_2O\rightarrow2Ag+CH_3COONH_4+2NH_4NO_3\)
0,123________________________________ 0,246______________________________
\(\rightarrow m_{Ag}=26,568\left(g\right)\)
Vậy chọn A

Đáp án C
Hướng dẫn nHCHO = nAg / 4 = 0,03 mol
=> nCH3OH phản ứng = nHCHO = 0,03 mol
=> H = nCH3OH phản ứng / nCH3OH ban đầu = 80%

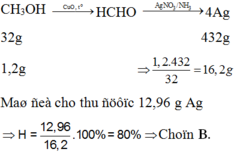
CH3OH → CuO , t o HCHO → AgNO 3 / NH 3 4Ag
32g 432g
1,2g ⇒ 1 , 2 . 432 32 = 16 , 2 g
Thu được 12,96 g Ag
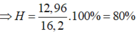
=> Chọn B.
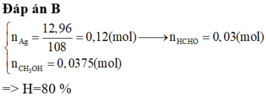
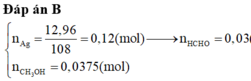
\(n_{C2H4\left(OH\right)2}=0,066\left(mol\right)\)
\(C_2H_4\left(OH\right)_2+CuO\rightarrow\left(CHO\right)_2+Cu+H_2O\)
\(\rightarrow n_{\left(CHO\right)2}=0,066\left(mol\right)\)
\(\rightarrow n_{CHO}=0,132\left(mol\right)\)
\(CHO+2AgNO_3+3NH_3+H_2O\rightarrow COONH_4+2Ag+2NH_4NO_3\)
\(\rightarrow n_{Ag}=0,264\left(mol\right)\)
\(\rightarrow m_{Ag}=28,512\left(g\right)\)