Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

Câu 1 : Qua nhận xét về phân tử khối và liên kết hidro trong mỗi hợp chất, ta có :
Thứ tự : Axit > Ancol > Este > Hidrocacbon
Ta thấy : Glyxin ở dạng ion lưỡng cực nên có nhiệt độ sôi cao hơn axit propionic
Vậy, theo chiều tăng dần nhiệt độ sôi, nhiệt độ nóng chảy là :
Glyxin > Axit propionic > Butan-1-ol >Metyl axetat > Butan

+ 1/2 X + NaOH dư:
\(n_{BaCO_3}=\dfrac{9,85}{197}=0,05mol\)
\(Ba^{2+}+HCO_3^-+OH^-\rightarrow BaCO_3\downarrow+H_2O\)
................0,05<-----------------0,05
\(\Rightarrow n_{HCO_3^-}=2.0,05=0,1mol\)
+ 1/2 X + NaHSO4 dư:
\(n_{BaSO_4}=\dfrac{17,475}{233}=0,075mol\)
\(HSO_4^-+HCO_3^-\rightarrow SO_4^{2-}+CO_2\uparrow+H_2O\)
\(Ba^{2+}+SO_4^{2-}\rightarrow BaSO_4\downarrow\)
\(Ba^{2+}+HSO_4^-\rightarrow BaSO_4\downarrow+H^+\) (nếu có)
\(\Rightarrow n_{Ba^{2+}}=2.0,075=0,15mol\)
Áp dụng định luật bảo toàn điện tích vào dung dịch X:
\(n_{HCO_3^-}+n_{Cl^-}=2n_{Ba^{2+}}+n_{Na^+}\)
\(\Rightarrow0,1+0,3=2.0,15+n_{Na^+}\Rightarrow n_{Na^+}=0,1mol\)
+ Đun nóng X:
\(2HCO_3^-\rightarrow^{t^0}CO_3^{2-}+CO_2\uparrow+H_2O\)
0,1------------>0,05
\(Ba^{2+}+CO_3^{2-}\rightarrow BaCO_3\downarrow\)
0,05<---0,05------->0,05
\(n_{Ba^{2+}}\text{còn}=0,15-0,05=0,1mol\)
=> Dung dịch X có chứa: 0,1 mol Ba2+; 0,1 Na+; 0,3 mol Cl-
\(\Rightarrow m_{\text{muối}}=137.0,1+23.0,1+35,5.0,3=26,65\left(gam\right)\)
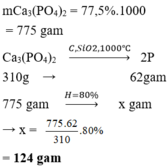
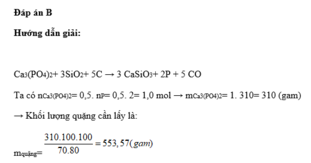
Chọn D