
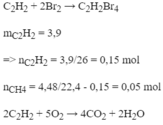
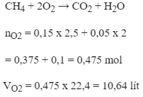
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

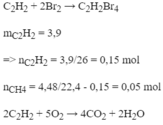
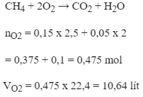

mtăng = mC2H4
=> \(n_{C_2H_4}=\dfrac{5,6}{28}=0,2\left(mol\right)\)
=> \(n_{CH_4}=\dfrac{8,96}{22,4}-0,2=0,2\left(mol\right)\)
PTHH: CH4 + 2O2 --to--> CO2 + 2H2O
0,2--->0,4
C2H4 + 3O2 --to--> 2CO2 + 2H2O
0,2---->0,6
=> VO2 = (0,4 + 0,6).22,4 = 22,4 (l)
=> Vkk = 22,4.5 = 112 (l)

\(m_{tăng}=m_{C_2H_2}=1,3\left(g\right)\\ \Rightarrow n_{C_2H_2}=\dfrac{1,3}{26}=0,05\left(mol\right)\\ \Rightarrow\%V_{\dfrac{C_2H_2}{A}}=\dfrac{0,05.22,4}{4,48}.100=25\%\\ \Rightarrow\%V_{\dfrac{CH_4}{A}}=100\%-25\%=75\%\)

\(C_2H_2+2Br_2\rightarrow C_2H_2Br_4\\ V_{CH_4}=4,48\left(l\right)\\ \Rightarrow V_{C_2H_2}=11,2-4,48=6,72\left(mol\right)\\ \Rightarrow\%V=\dfrac{6,72}{11,2}.100=60\%\)

\(n_{hh}=\dfrac{3,36}{22,4}=0,15mol\)
\(n_{Br_2}=\dfrac{2,4}{160}=0,015mol\)
\(C_2H_2+2Br_2\rightarrow C_2H_2Br_4\)
0,0075 0,015 ( mol )
\(V_{C_2H_2}=0,0075.22,4=0,168l\)
\(V_{CH_4}=3,36-0,168=3,192l\)
\(\%V_{C_2H_2}=\dfrac{0,168}{3,36}.100=5\%\)
\(\%V_{CH_4}=100\%-5\%=95\%\)