
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.



\(n_{KMnO_4}=\dfrac{31,6}{158}=0,2\left(mol\right)\)
\(2KMnO_4->K_2MnO_4+MnO_2+O_2\)
2......................1................1...................1
0,2................0,1..............0,1....................0,1
\(m_{K_2MnO_4}=0,1.197=19,7\left(g\right)\\ m_{MnO_2}=0,1.87=8,7\left(g\right)\\ m_{O_2}=0,1.32=3,2\left(g\right)\)

a. \(PTHH:3H_2+Fe_2O_3\underrightarrow{t^o}2Fe+3H_2O\)
b. \(n_{Fe_2O_3}=\dfrac{m_{Fe_2O_3}}{M_{Fe_2O_3}}=\dfrac{4}{160}=0,025\left(mol\right)\)
\(PTHH:3H_2+Fe_2O_3\underrightarrow{t^o}2Fe+3H_2O\)
Mol : 3 : 1 : 2 : 3
Mol : 0,075 ← 0,025 → 0,05 → 0,075
\(\Rightarrow n_{H_2}=0,075\left(mol\right)\)
\(\Rightarrow V_{H_2}=n_{H_2}.22,4=0,075.22,4=1,68\left(l\right)\)
c. Từ câu b. \(\Rightarrow n_{Fe}=0,05\left(mol\right)\)
\(\Rightarrow m_{Fe}=n_{Fe}.M_{Fe}=0,05.56=2,8\left(g\right)\)




\(Zn\rightarrow H_2\rightarrow HCl\rightarrow H_2O\rightarrow O_2\)
\(Zn+2HCl\rightarrow ZnCl_2+H_2\)
\(H_2+Cl_2\underrightarrow{^{^{as}}}2HCl\)
\(ZnO+2HCl\rightarrow ZnCl_2+H_2O\)
\(2H_2O\underrightarrow{^{^{dp}}}2H_2+O_2\)
\(PTHH:Zn+2HCl\rightarrow ZnCl_2+H_2\)
\(PTHH:2H_2+O_2\underrightarrow{t^o}2H_2O\)


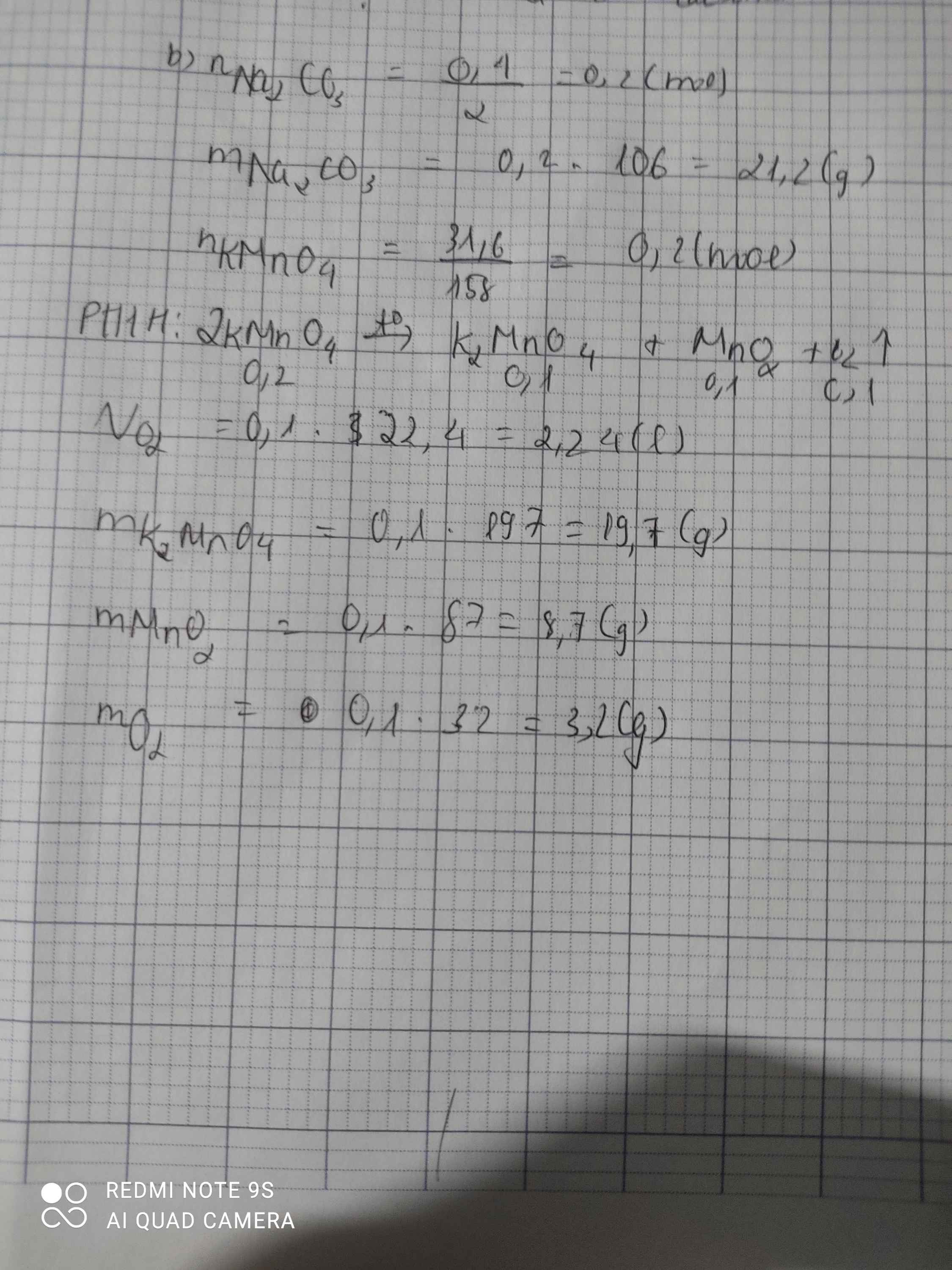



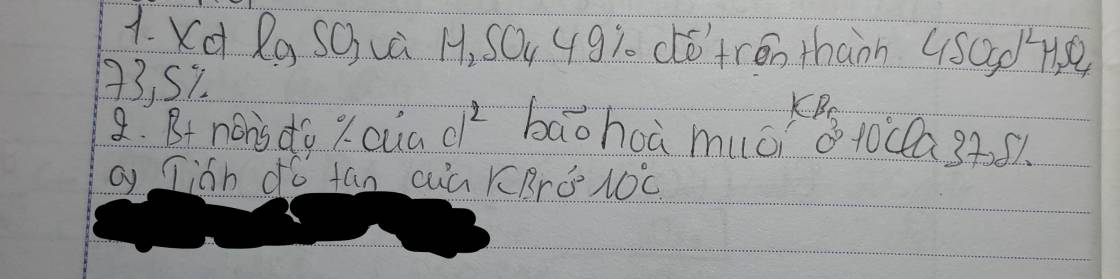 giải giúp mik vs ạ mik đang cần gấp!!!
giải giúp mik vs ạ mik đang cần gấp!!!
gggggggggggggggggggggggggggggggggggggggggggnv