Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{O_2}=\dfrac{5.6}{22.4}=0.25\left(mol\right)\)
\(n_{H_2}=\dfrac{10.08}{22.4}=0.45\left(mol\right)\)
\(n_{Fe}=a\left(mol\right),n_{Al}=b\left(mol\right)\)
\(3Fe+2O_2\underrightarrow{^{^{t^0}}}Fe_3O_4\)
\(a.......\dfrac{2a}{3}\)
\(4Al+3O_2\underrightarrow{^{^{t^0}}}2Al_2O_3\)
\(b.......\dfrac{3b}{4}\)
\(n_{O_2}=\dfrac{2a}{3}+\dfrac{3b}{4}=0.25\left(mol\right)\left(1\right)\)
\(Fe+2HCl\rightarrow FeCl_2+H_2\)
\(2Al+6HCl\rightarrow2AlCl_3+3H_2\)
\(n_{H_2}=a+1.5b=0.45\left(mol\right)\left(2\right)\)
\(\left(1\right),\left(2\right):a=0.15,b=0.2\)
\(m_{Fe}=0.15\cdot56=8.4\left(g\right)\)
\(m_{Al}=0.2\cdot27=5.4\left(g\right)\)
\(\%m_{Fe}=\dfrac{8.4}{8.4+5.4}\cdot100\%=60.8\%\)
\(\%m_{Al}=100-60.8=39.2\%\)

b.\(n_{HCl}=\dfrac{m_{HCl}}{M_{HCl}}=\dfrac{29,2}{36,5}=0,8mol\)
Gọi \(\left\{{}\begin{matrix}n_{Al}=x\\n_{Mg}=y\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}m_{Al}=27x\\m_{Mg}=24y\end{matrix}\right.\)
\(2Al+6HCl\rightarrow2AlCl_3+3H_2\)
x 3x ( mol )
\(Mg+2HCl\rightarrow MgCl_2+H_2\)
y 2y ( mol )
Ta có:
\(\left\{{}\begin{matrix}27x+24y=7,8\\3x+2y=0,8\end{matrix}\right.\) \(\Leftrightarrow\left\{{}\begin{matrix}x=0,2\\y=0,1\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}m_{Al}=0,2.27=5,4g\\m_{Mg}=0,1.24=2,4g\end{matrix}\right.\)

a) Gọi số mol của Mg là x, của Al là y \(\left(x,y>0\right)\)
Ta có: \(24x+27y=7,8\) (*)
PTHH:
\(Mg+H_2SO_4\rightarrow MgSO_4+H_2\uparrow\) (1)
\(2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\uparrow\) (2)
\(n_{H_2\left(1\right)+\left(2\right)}=\dfrac{9,916}{24,79}=0,4\left(mol\right)\)
Theo PTHH: \(n_{H_2\left(1\right)}=n_{Mg}=x\left(mol\right)\); \(n_{H_2\left(2\right)}=\dfrac{3}{2}n_{Al}=\dfrac{3}{2}y\left(mol\right)\)
\(\Rightarrow x+\dfrac{2}{3}y=0,4\) (**)
Từ (*) và (**) ta có hệ:
\(\left\{{}\begin{matrix}24x+27y=7,8\\x+\dfrac{3}{2}y=0,4\end{matrix}\right.\)
Giải hệ ta được: \(\left\{{}\begin{matrix}x=0,1\\y=0,2\end{matrix}\right.\)
\(\Rightarrow\%m_{Al}=\dfrac{27\cdot0,2\cdot100}{7,8}\approx69\%\)
\(\Rightarrow\%m_{Mg}=100\%-69\%=21\%\)
b) \(n_{MgSO_4}=n_{Mg}=0,1\left(mol\right)\); \(n_{Al_2\left(SO_4\right)_3}=\dfrac{1}{2}n_{Al}=0,1\left(mol\right)\)
Tổng khối lượng muối là:
\(m_{MgSO_4}+m_{Al_2\left(SO_4\right)_3}=0,1\cdot120+0,1\cdot342=46,2\)

Gọi: \(\left\{{}\begin{matrix}n_{Al}=x\left(mol\right)\\n_{Fe}=y\left(mol\right)\end{matrix}\right.\) ⇒ 27x + 56y = 5,5 (1)
PT: \(2Al+6HCl\rightarrow2AlCl_3+3H_2\)
\(Fe+2HCl\rightarrow FeCl_2+H_2\)
Theo PT: \(n_{H_2}=\dfrac{3}{2}n_{Al}+n_{Fe}=\dfrac{3}{2}x+y=\dfrac{4,48}{22,4}=0,2\left(mol\right)\left(2\right)\)
Từ (1) và (2) \(\Rightarrow\left\{{}\begin{matrix}x=0,1\left(mol\right)\\y=0,05\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}\%m_{Al}=\dfrac{0,1.27}{5,5}.100\%\approx49,09\%\\\%m_{Fe}\approx50,91\%\end{matrix}\right.\)

Sửa đề : 13.9 (g)
\(n_{Al}=a\left(mol\right),n_{Fe}=b\left(mol\right)\)
\(\Rightarrow m=27a+56b=13.9\left(1\right)\)
\(n_{H_2}=\dfrac{7.84}{22.4}=0.35\left(mol\right)\)
\(2Al+6HCl\rightarrow2AlCl_3+3H_2\)
\(Fe+2HCl\rightarrow FeCl_2+H_2\)
\(n_{H_2}=1.5a+b=0.35\left(2\right)\)
\(\left(1\right),\left(2\right):a=0.1,b=0.2\)
\(\%Al=\dfrac{0.1\cdot27}{13.9}\cdot100\%=19.42\%\)
\(\%Fe=100-19.42=80.58\%\)

a, PT: \(2Al+6HCl\rightarrow2AlCl_3+3H_2\)
\(Mg+2HCl\rightarrow MgCl_2+H_2\)
b, Gọi: \(\left\{{}\begin{matrix}n_{Al}=x\left(mol\right)\\n_{Mg}=y\left(mol\right)\end{matrix}\right.\) ⇒ 27x + 24y = 7,8 (1)
Ta có: m dd tăng = mKL - mH2 ⇒ mH2 = 7,8 - 7 = 0,8 (g)
\(\Rightarrow n_{H_2}=\dfrac{0,8}{2}=0,4\left(mol\right)\)
Theo PT: \(n_{H_2}=\dfrac{3}{2}n_{Al}+n_{Mg}=\dfrac{3}{2}x+y=0,4\left(mol\right)\left(2\right)\)
\(\Rightarrow\left\{{}\begin{matrix}x=0,2\left(mol\right)\\y=0,1\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}\%m_{Al}=\dfrac{0,2.27}{7,8}.100\%\approx69,23\%\\\%m_{Mg}\approx30,77\%\end{matrix}\right.\)

Gọi \(\left\{{}\begin{matrix}n_{Al}=x\left(mol\right)\\n_{Fe}=y\left(mol\right)\end{matrix}\right.\)
\(n_{H_2}=\dfrac{4,48}{22,4}=0,2mol\)
\(2Al+6HCl\rightarrow2AlCl_3+3H_2\)
\(Fe+2HCl\rightarrow FeCl_2+H_2\)
Theo pt: \(\Rightarrow\left\{{}\begin{matrix}3x+y=0,2\\27x+56y=5,5\end{matrix}\right.\Rightarrow\left\{{}\begin{matrix}x=\dfrac{19}{470}\\y=\dfrac{37}{470}\end{matrix}\right.\)
\(\%m_{Al}=\dfrac{\dfrac{19}{470}\cdot27}{5,5}\cdot100\%=19,84\%\)
\(\%m_{Fe}=100\%-19,84\%=80,16\%\)

Gọi nFe = a (mol); nAl = b (mol)
=> 56a + 27b = 11 (1)
nH2 = 8,96/22,4 = 0,4 (mol)
PTHH:
Fe + 2HCl -> FeCl2 + H2
a ---> 2a ---> a ---> a
2Al + 6HCl -> 2AlCl3 + 3H2
b ---> 1,5b ---> b ---> b
=> a + 1,5b = 0,4 (2)
Từ (1)(2) => a = 0,1 (mol); b = 0,15 (mol)
mFe = 0,1 . 56 = 5,6 (g)
mAl = 0,2 . 27 = 5,4 (g)
THAM KHẢO :
Fe + 2HCl -> FeCl2 + H2 (1)
a) 2Al + 6HCl -> 2AlCl3 + 3H2 (2)
Gọi khối lượng Fe là x(g) (0<x<11) => nFe = x/56 (mol)
Thì mAl là 11-x(g) => nAl = (11-x)/27 (mol)
nH2 = 8,96/22,4 = 0,4 (mol)
Theo PT (1) ta có: nH2 = nFe = x/56 (mol)
Theo PT (2) ta có: nH2 = 3/2 nAl = 3/2 . (11-x)/27 = (11-x)/18 (mol)
Theo đề bài, nH2 thu được là 0,4(mol) nên ta có:
x/56 + (11-x)/18 = 0,4
<=> 18x +56(11-x) = 403,2
<=> x = 5,6 (g)
Do đó: mFe = 5,6(g) => nFe = 5,6/56 = 0,1 (mol)
mAl = 11-5,6 = 5,4(g) => nAl = 5,4/27 = 0,2 (mol)
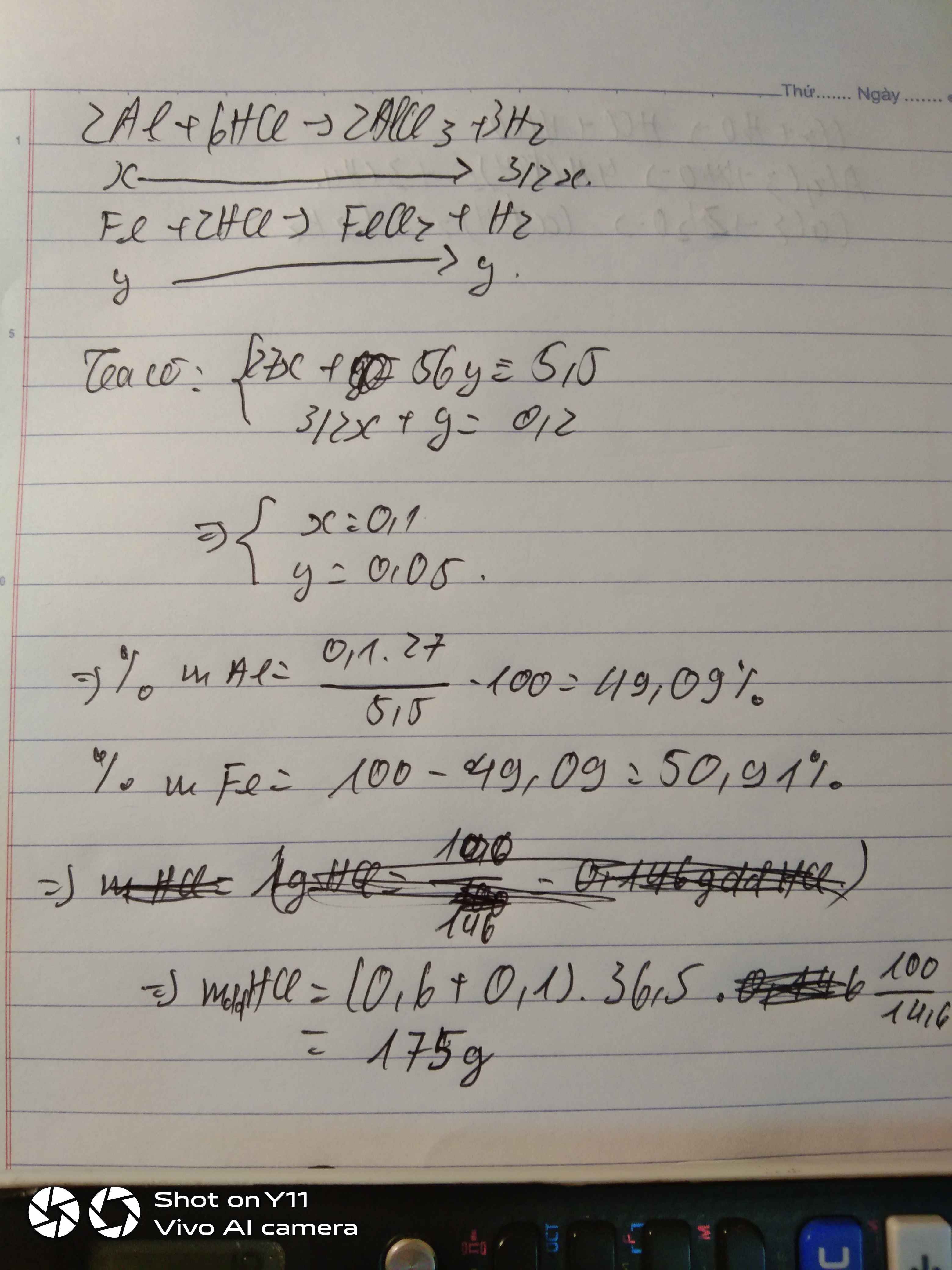
Gọi số mol Al, Mg là a, b (mol)
=> 27a + 24b = 7,8 (1)
\(n_{HCl}=\dfrac{29,2}{36,5}=0,8\left(mol\right)\)
PTHH: 2Al + 6HCl --> 2AlCl3 + 3H2
a---->3a
Mg + 2HCl --> MgCl2 + H2
b-->2b
=> 3a + 2b = 0,8 (2)
(1)(2) => a = 0,2 (mol); b = 0,1 (mol)
\(\left\{{}\begin{matrix}m_{Al}=0,2.27=5,4\left(g\right)\\m_{Mg}=0,1.24=2,4\left(g\right)\end{matrix}\right.\)