Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

PTHH: Fe + 2HCl --> FeCl2 + H2
FeS + 2HCl --> FeCl2 + H2S
=> \(n_{Fe}+n_{FeS}=n_{H_2}+n_{H_2S}=\dfrac{5,6}{22,4}=0,25\left(mol\right)\)
Và 56.nFe + 88.nFeS = 18,8
=> \(\left\{{}\begin{matrix}n_{Fe}=0,1\left(mol\right)\\n_{FeS}=0,15\left(mol\right)\end{matrix}\right.\)
Bảo toàn S: nCaSO3 = 0,15 (mol)
=> m = 0,15.120 = 18 (g)
=> B

a)
Gọi số mol Fe, Al là a, b (mol)
=> 56a + 27b = 19,3 (1)
\(n_{H_2}=\dfrac{14,56}{22,4}=0,65\left(mol\right)\)
PTHH: Fe + H2SO4 --> FeSO4 + H2
a--->a---------------->a
2Al + 3H2SO4 --> Al2(SO4)3 + 3H2
b---->1,5b------------------->1,5b
=> a + 1,5b = 0,65 (2)
(1)(2) => a = 0,2 (mol); b = 0,3 (mol)
mFe = 0,2.56 = 11,2 (g); mAl = 0,3.27 = 8,1 (g)
b)
\(n_{H_2SO_4}=0,65\left(mol\right)\)
=> \(C_{M\left(dd.H_2SO_4\right)}=\dfrac{0,65}{0,2}=3,25M\)

\(n_{KMnO_4}=0,1\left(mol\right)\)
\(PTHH:2KMnO_4\underrightarrow{t^o}K_2MnO_4+MnO_2+O_2\)
\(\Rightarrow n_{O_2}=\dfrac{0,1}{2}=0,05\left(mol\right)\)
Đặt \(\left\{{}\begin{matrix}n_{Cu}=a\left(mol\right)\\n_{Fe}=b\left(mol\right)\end{matrix}\right.\)
Bảo toàn e:\(\Rightarrow2a+3b=0,5\)
Mặt khác: \(64a+56b=13,6-0,05.32=12\)
\(\Rightarrow\left\{{}\begin{matrix}a=0,1\\b=0,1\end{matrix}\right.\)
\(\Rightarrow\%m_{Fe}=\dfrac{0,1.56}{12}.100\%=46,67\left(\%\right)\)
Tại sao lại có 2a+3b=0,5 ạ ?
Qúa trình nhường e của Fe diễn ra ntn ạ ?

\(n_{H_2}=\dfrac{4,48}{22,4}=0,2\left(mol\right)\)
PTHH: Fe + 2HCl --> FeCl2 + H2
0,2<-------------------0,2
=> mFe = 0,2.56 = 11,2 (g)
\(n_{CuS}=\dfrac{9,6}{96}=0,1\left(mol\right)\)
PTHH: Cu(NO3)2 + H2S --> CuS + 2HNO3
0,1<---0,1
FeS + 2HCl --> FeCl2 + H2S
0,1<---------------------0,1
=> mFeS = 0,1.88 = 8,8 (g)
=> m = 11,2 + 8,8 = 20 (g)
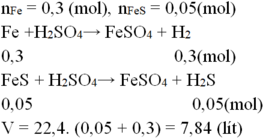


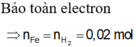
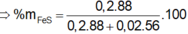
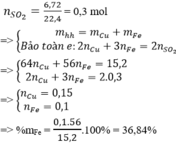
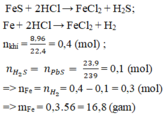
Câu này 200 gam dung dịch mới đúng, 400 ra nghiệm âm
Gọi số mol Fe là x; FeS là y
\(\Rightarrow56x+88y=14,4\)
\(Fe+H_2SO_4\rightarrow FeSO_4+H_2\)
\(FeS+H_2SO_4\rightarrow FeSO_4+H_2S\)
\(m_{H2SO_4}=200.9,8\%=19,6\left(g\right)\Rightarrow m_{H2SO4}=\frac{19,6}{98}=0,2\left(mol\right)\)
Theo phản ứng: \(n_{H2SO4}=x+y=0,2\left(mol\right)\)
Giải được: \(x=y=0,1\left(mol\right)\)
\(\Rightarrow m_{Fe}=56.0,1=5,6\left(g\right);m_{FeS}=88.0,1=8,8\left(g\right)\)
\(\Rightarrow n_{H2}=n_{Fe}=0,1\left(mol\right);n_{H2S}=n_{FeS}=0,1\left(mol\right)\)
Vì % số mol = % thể tích \(\Rightarrow\%V_{H2}=\%V_{H2S}=50\%\)
BTKL: m rắn + mH2SO4 =m dung dịch X + m khí
\(\Rightarrow m_{dd_X}=14,4+200-0,1.2-0,1.34=210,8\left(g\right)\)\(n_{FeSO_4}=n_{Fe}=0,2\left(mol\right)\Rightarrow m_{FeSO_4}=0,2.\left(56+96\right)=30,4\left(g\right)\)\(\Rightarrow C\%_{FeSO4}=\frac{30,4}{210,8}=14,4\%\)