Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a) \(n_{H_2}=\dfrac{3,36}{22,4}=0,15\left(mol\right)\)
PTHH: Fe + 2HCl --> FeCl2 + H2
____0,15<--0,3<--------------0,15
=> mFe = 0,15.56 = 8,4 (g)
=> \(\left\{{}\begin{matrix}\%Fe=\dfrac{8,4}{21,2}.100\%=39,62\%\\\%Cu=\dfrac{21,2-8,4}{21,2}.100\%=60,38\%\end{matrix}\right.\)
b) mHCl = 0,3.36,5 = 10,95(g)
=> \(m_{ddHCl}=\dfrac{10,95.100}{3,65}.100\%=300\left(g\right)\)

a:
\(n_{HCl}=0.2\cdot1=0.2\left(mol\right)\)
\(Fe+2HCl\rightarrow FeCl_2+H_2\uparrow\)
b: \(Fe+2HCl\rightarrow FeCl_2+H_2\uparrow\)
0,1 0,2 0,1 0,1
\(m_{Fe}=0.1\cdot56=5.6\left(g\right)\)
c: \(m_{FeCl_2}=0.1\cdot\left(56+35.5\cdot2\right)=12.7\left(g\right)\)
d: \(V_{H_2}=0.1\cdot22.4=2.24\left(lít\right)\)

a)Pt : Fe + 2HCL\(\rightarrow\)FeCL2 + H2
b ) nH2 = \(\frac{3,36}{22,4}\)= 0,15 (mol)
Theo pt ta lại có: nH2= n Fe=0,15mol
\(\Rightarrow\)mFe=0,15 . 56 = 8,4(g)
c) Đổi ; 100ml = 0,1 l
Theo pt ta lại có :nHCL= 2nH2= 0,15 . 2 = 0,3 (mol)
CMHCL= \(\frac{0,3}{0,1}\) = 3

a, PT: \(Fe+2HCl\rightarrow FeCl_2+H_2\)
b, Ta có: \(n_{H_2}=\dfrac{3,36}{22,4}=0,15\left(mol\right)\)
Theo PT: \(n_{Fe}=n_{H_2}=0,15\left(mol\right)\)
\(\Rightarrow m_{Fe}=0,15.56=8,4\left(g\right)\)

a, \(Zn+2HCl\rightarrow ZnCl_2+H_2\)
b, \(n_{H_2}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\)
Theo PT: \(n_{Zn}=n_{H_2}=0,3\left(mol\right)\)
\(\Rightarrow m_{Zn}=0,3.65=19,5\left(g\right)\)
c, \(n_{HCl}=2n_{H_2}=0,6\left(mol\right)\)
\(\Rightarrow C_{M_{HCl}}=\dfrac{0,6}{0,1}=6\left(M\right)\)
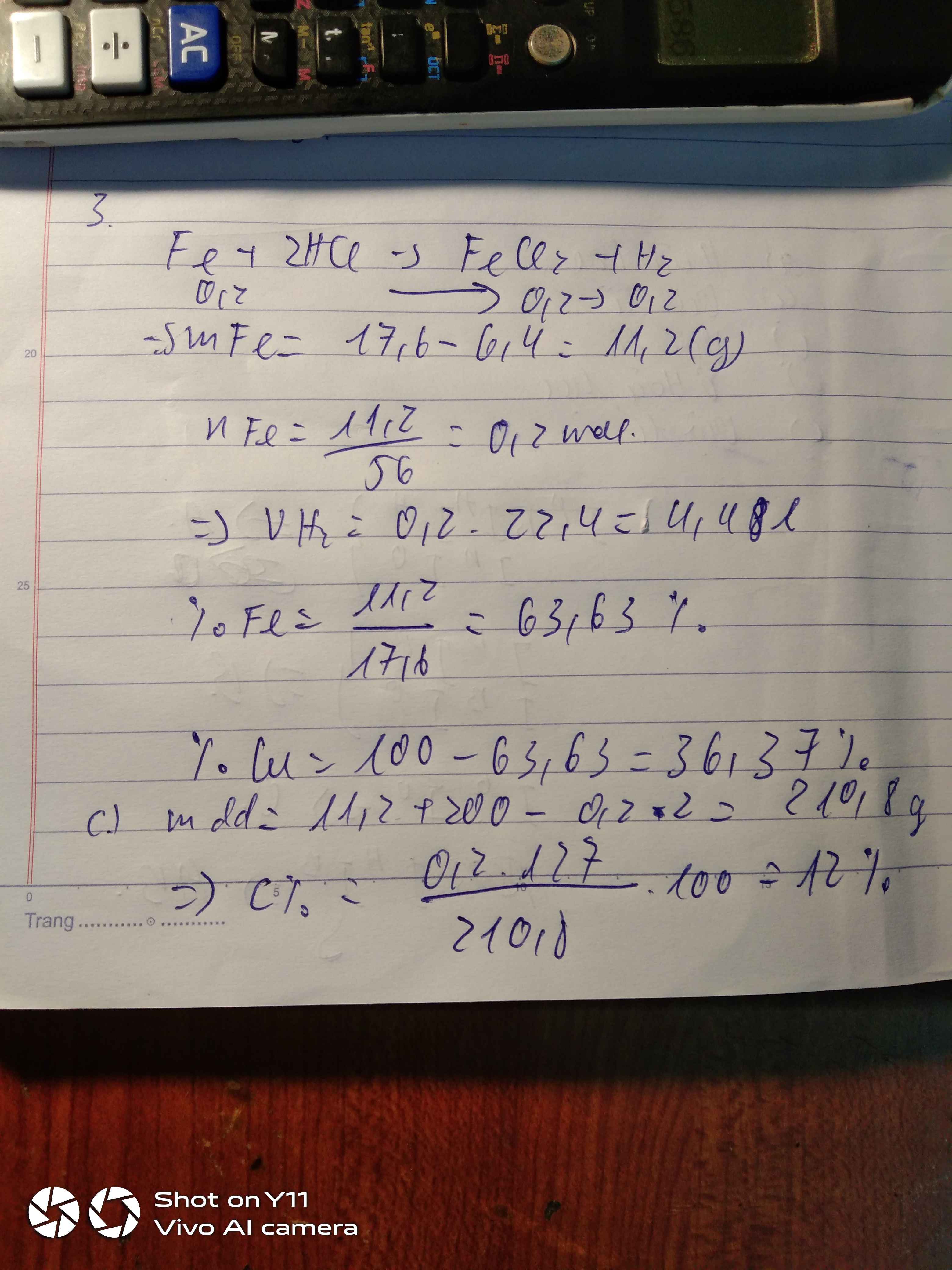
a) \(n_{H_2}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\)
PTHH: \(Fe+2HCl\rightarrow FeCl_2+H_2\)
nFe = nH2 = 0,3 (mol)
\(\Rightarrow m_{Fe}=0,3.56=16,8\left(g\right)\)
b) nHCl = 2.nH2 = 0,6 (mol)
\(\Rightarrow V_{ddHCl}=\dfrac{0,6}{0,3}=2\left(l\right)\)
c) \(n_{FeCl_2}=n_{H_2}=0,3\left(mol\right)\)
\(\Rightarrow C_{M\left(FeCl_2\right)}=\dfrac{0,3}{2}=0,15M\)
\(Fe+2HCl\rightarrow FeCl_2+H_2\\ a,n_{H_2}=\dfrac{6,72}{22,4}=0,3mol\\ n_{Fe}=n_{FeCl_2}=n_{H_2}=0,3mol\\ m_{Fe}=0,3.56=16,8g\\ b,n_{HCl}=2n_{H_2}=0,3.2=0,6mol\\ V_{ddHCl}=\dfrac{0,6}{0,3}=2l\\ c,C_{M_{FeCl_2}}=\dfrac{0,3}{2}=0,15M\)