Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a) PTHH: Na2O + H20 -> 2NaOH
số mol Na20 = 0,25 (mol)
=> số mol NaOH = 0,5 mol.
Nôngd độ mol NaOH = 0,5 / 0,5 = 1 M
b) PTHH: H2SO4 + 2NaOH -> Na2SO4 + 2H2O
số mol H2SO4 = 1/2 số mol NaOH = 0,25 mol
C% H2SO4 = mH2SO4 / m ddH2SO4 . 100%
=> m ddH2SO4= 122,5 g
D=m/V => V= 107,5 ml
Số mol Na2O = 15,5:62 = 0,25 mol
a) Khi cho Na2O xảy ra phản ứng, tạo thành phản ứng dung dịch có chất tan là NaOH.
Na2O + H2O → 2NaOH
Phản ứng: 0,25 → 0,05 (mol)
500 ml = = 0,5 lít; CM, NaOH =
= 1M.
b) Phương trình phản ứng trung hòa dung dịch:
2NaOH + H2SO4 → Na2SO4 + 2H2O
Phản ứng: 0, 5 → 0,25 0,25 (mol)
mH2SO4 = 0,25x98 = 24,5 g
mdd H2SO4 = = 122,5 g
mdd, ml = =
≈ 107,5 ml

Ta có: \(n_{Na_2O}=\dfrac{31}{62}=0,5\left(mol\right)\)
\(PTHH:Na_2O+H_2O--->2NaOH\)
Theo PT: \(n_{NaOH}=2.n_{Na_2O}=2.0,5=1\left(mol\right)\)
\(\Rightarrow C_{M_{NaOH}}=\dfrac{1}{0,5}=2M\)
`Na_2O + H_2O -> 2NaOH`
`n_{Na_2O} = (31)/(62) = 0,5` `mol`
`n_{NaOH} = 2 . n_{Na_2O} = 1` `mol`
`C_{M_(NaOH)} = 1/(0,5) = 2` `M`

bài 1:
- Trích mỗi chất 1 ít làm mẫu thử
- Nhỏ vài giọt các dd trên vào mẫu giấy quỳ tím
+ quỳ tím chuyển sang xanh : Ba(OH)2 , NaOH (I)
+ Không có hiện tượng gì : NaCl , Na2SO4 (II)
- Trích từng chất dd ở nhóm I vào nhóm II , thấy xuất hiện kết tủa trắng thì đó là Ba(OH)2 và Na2SO4
Ba(OH)2 + Na2SO4 → BaSO4↓ + 2NaOH
- Hai dd còn lại là NaCl(không làm quỳ tím đổi màu)
Và NaOH ( quỳ làm tím hóa xanh )

\(\text{1) Na2O+H2O->2NaOH}\)
Ta có :
\(\text{nNaOH=2x12,4/62+1x0,2=0,6(mol)}\)
\(\Rightarrow\text{CMNaOH=0,6/1=0,6(M)2}\)
buithianhtho, Pham Van Tien, Duong Le, Nguyễn Thị Kiều, Dương Chung, Linh, Luân Trần, Arakawa Whiter, Trần Quốc Toàn, Đặng Anh Huy 20141919, Nguyễn Nhật Anh, Trần Hữu Tuyển, Phùng Hà Châu, Quang Nhân, Hoàng Tuấn Đăng, Nguyễn Trần Thành Đạt, Nguyễn Thị Minh Thương , Nguyễn Anh Thư,...

a) Na2O +H2O--->2NaOH(1)
NaOH +HCl-->NaCl2 +H2(2)
b) Ta có
n\(_{Na2O}=\frac{12,4}{62}=0,2\left(mol\right)\)
Theo pthh1
n\(_{NaOH}=2n_{Na2O}=0,4\left(mol\right)\)
Theo pthh2
n\(_{HCl}=n_{NaOH}=0,4\left(mol\right)\)
Tính nồng độ mol hợp lý hơn
CM\(_{HCl}=\frac{0,4}{0,2}=2\left(M\right)\)
c)CM\(_{NaOH}=\frac{0,2}{0,2}=1\left(M\right)\)
Theo pthh2
n\(_{NaCl}=n_{NaOH}=0,2\left(mol\right)\)
C\(_M=\frac{0,2}{0,2}=1\left(M\right)\)

1)
$n_{Na_2O} = \dfrac{6,2}{62} = 0,1(mol)$
$Na_2O + H_2O \to 2NaOH$
$n_{NaOH} = 2n_{Na_2O} = 0,2(mol)$
$m_{dd} = 6,2 + 193,8 = 200(gam) \Rightarrow C\%_{NaOH} = \dfrac{0,2.40}{200}.100\% = 4\%$
2)
$n_{K_2O} = \dfrac{23,5}{94} = 0,25(mol)$
$K_2O + H_2O \to 2KOH$
$n_{KOH} = 2n_{K_2O} = 0,5(mol) \Rightarrow C_{M_{KOH}} = \dfrac{0,5}{0,5} = 1M$
3) $n_{Na_2O} = \dfrac{12,4}{62} = 0,2(mol)$
$Na_2O + H_2O \to 2NaOH$
$n_{NaOH} = 2n_{Na_2O} = 0,4(mol)$
$C_{M_{NaOH}} = \dfrac{0,4}{0,5} =0,8M$
4)
$Na_2SO_3 + 2HCl \to 2NaCl +S O_2 + H_2O$
Theo PTHH :
$n_{SO_2} = n_{Na_2SO_3} = \dfrac{12,6}{126} = 0,1(mol)$
$V_{SO_2} = 0,1.22,4 = 2,24(lít)$
5) $n_{CaO} = \dfrac{5,6}{56} = 0,1(mol)$
$CaO + 2HCl \to CaCl_2 + H_2O$
Theo PTHH :
$n_{HCl} = 2n_{CaO} = 0,2(mol) \Rightarrow m_{dd\ HCl} = \dfrac{0,2.36,5}{14,6\%} = 50(gam)$
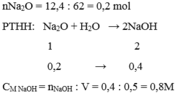
\(n_{Na_2O}=\dfrac{12,4}{62}=0,2mol\)
\(Na_2O+H_2O\rightarrow2NaOH\)
0,2 \(\rightarrow\) 0,2 \(\rightarrow\) 0,4
\(C_{M_{NaOH}}=\dfrac{0,4}{\dfrac{500}{1000}}=0,8M\)