Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

Sửa đề: 9,2 gam Na
\(a,n_{Na_2O}=\dfrac{9,2}{23}=0,4\left(mol\right)\)
PTHH: \(Na_2O+H_2O\rightarrow2NaOH\)
0,4------------------>0,8
\(\rightarrow C_{M\left(NaOH\right)}=\dfrac{0,8}{0,5}=1,6M\)
\(b,n_{K_2O}=\dfrac{37,6}{94}=0,4\left(mol\right)\)
PTHH: \(K_2O+H_2O\rightarrow2KOH\)
0,4----------------->0,8
\(\rightarrow C\%_{KOH}=\dfrac{0,8.56}{362,4+37,6}.100\%=11,2\%\)

a, \(C\%_{KCl}=\dfrac{20}{20+60}.100\%=25\%\)
b, \(C\%=\dfrac{40}{40+150}.100\%\approx21,05\%\)
c, \(C\%_{NaOH}=\dfrac{60}{60+240}.100\%=20\%\)
d, \(C\%_{NaNO_3}=\dfrac{30}{30+90}.100\%=25\%\)
e, \(m_{NaCl}=150.60\%=90\left(g\right)\)
f, \(m_{ddA}=\dfrac{25}{10\%}=250\left(g\right)\)
g, \(n_{NaOH}=120.20\%=24\left(g\right)\)
Gọi: nNaOH (thêm vào) = a (g)
\(\Rightarrow\dfrac{a+24}{a+120}.100\%=25\%\Rightarrow a=8\left(g\right)\)

a/
\(n_{Na_2O}=\dfrac{9,3}{62}=0,15\left(mol\right)\)
\(Na_2O+H_2O\rightarrow2NaOH\)
0,15 0,3 (mol)
\(m_{NaOH}=0,3.40=12\left(g\right)\)
\(m_A=90,7+9,3=100\left(g\right)\)
\(C\%_{NaOH}=\dfrac{12}{100}.100\%=12\%\)
b/
m\(_{FeSO_4}=\dfrac{16.200}{100}=32\left(g\right)\)
\(\rightarrow m_{FeSO_4}=\dfrac{32}{152}=\dfrac{4}{19}\left(mol\right)\)
\(2NaOH+FeSO_4\rightarrow Na_2SO_4+Fe\left(OH\right)_2\downarrow\)
bđ: 0,3 \(\dfrac{4}{19}\) 0 0 (mol)
pư: 0,3 0,15 0,15 0,15 (mol)
dư: 0 \(\dfrac{23}{380}\) (mol)
\(m_{Fe\left(OH\right)_2}=0,15.90=13,5\left(g\right)\)
\(m_C=100+200-13,5=286,5\left(g\right)\)
\(m_{Na_2SO_4}=0,15.142=21,3\left(g\right)\)
\(\rightarrow C\%_{Na_2SO_4}=\dfrac{21,3}{286,5}.100\%\approx7,4\%\)
\(m_{FeSO_4\left(dư\right)}=\dfrac{23}{380}.152=9,2\left(g\right)\)
\(\rightarrow C\%_{FeSO_4\left(dư\right)}=\dfrac{9,2}{286,5}.100\%\approx3,2\%\)

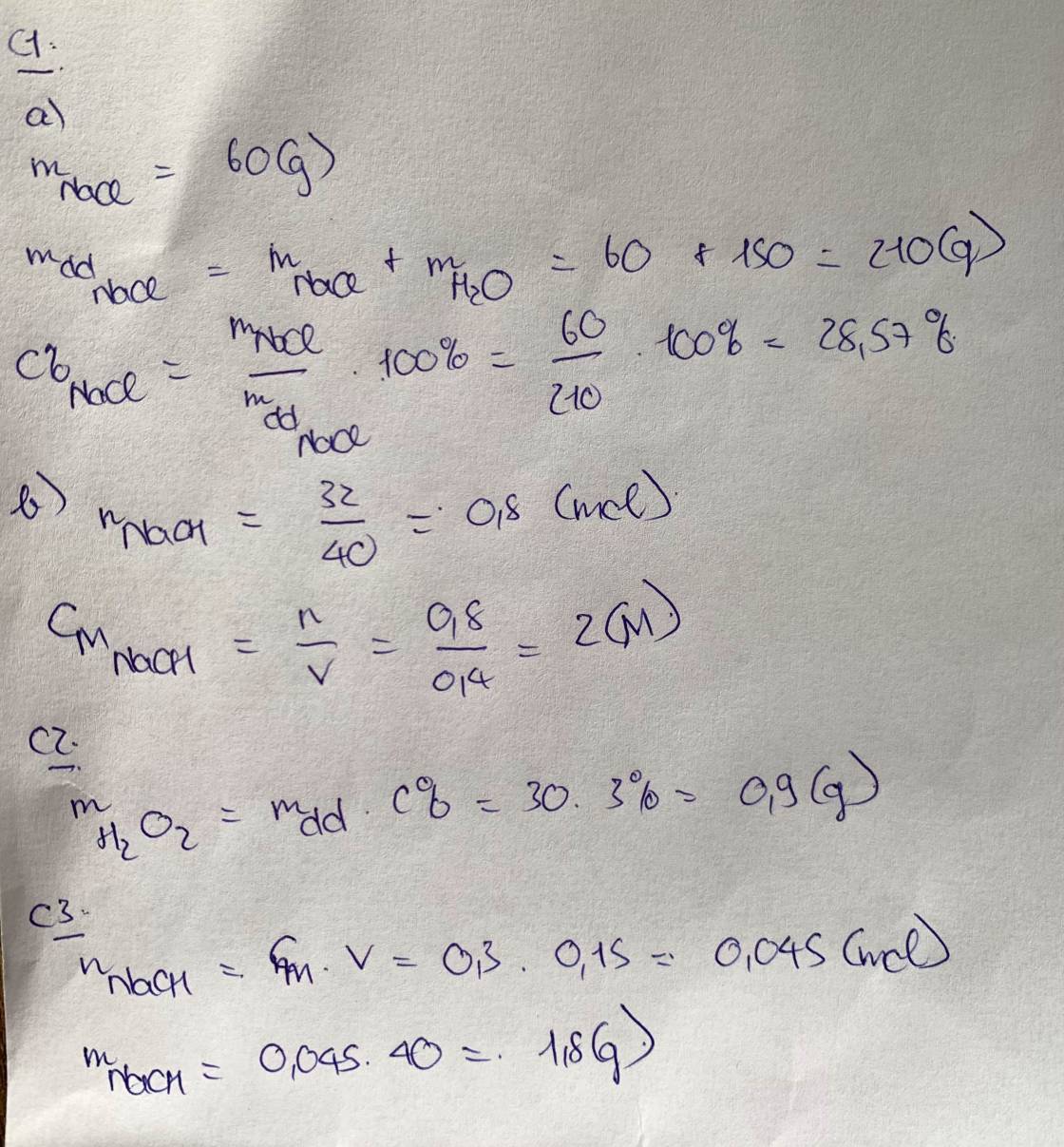
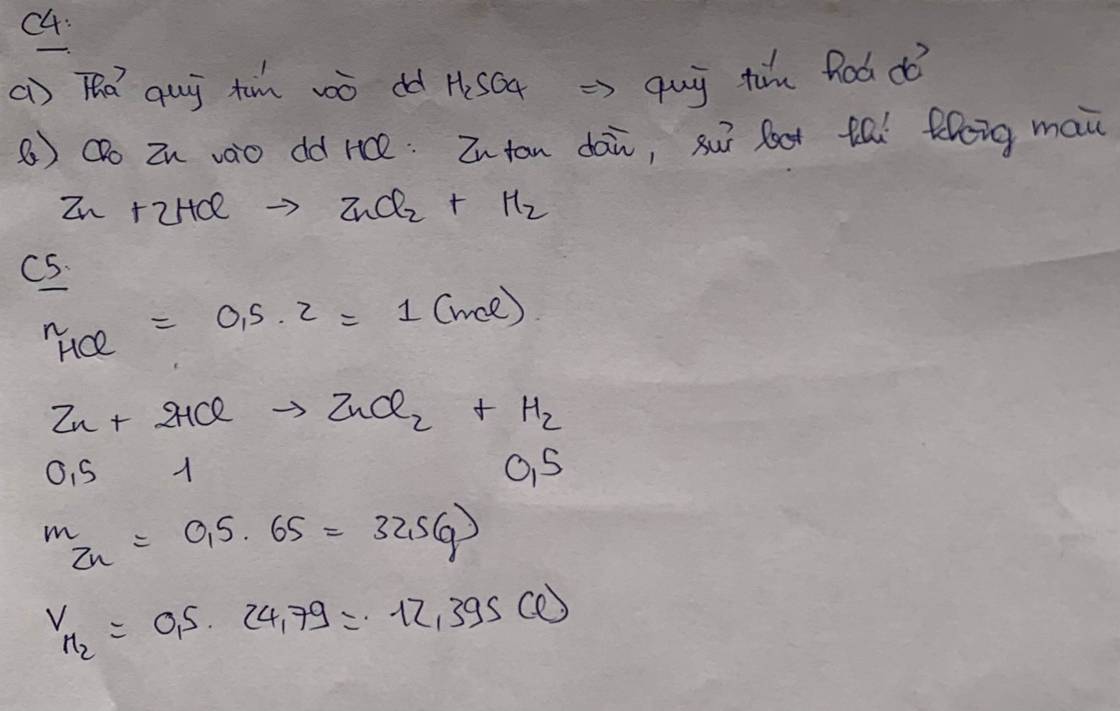

Na2O=0,5 mol
Na2O+H2O->2NaOH
0,5-----------------1 mol
ta có m NaOH=1.40+40=80g
=>C%=\(\dfrac{80}{431}100=18,561\%\)
Bạn ơi sao lại 1.40+40 vậy ạ?