Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

Bảo toàn nguyên tố: \(n_{H_2SO_4}=n_{H_2}=\dfrac{11,2}{22,4}=0,5\left(mol\right)\)
\(\Rightarrow\left\{{}\begin{matrix}m_{H_2}=0,5\cdot2=1\left(g\right)\\m_{H_2SO_4}=0,5\cdot98=49\left(g\right)\end{matrix}\right.\)
Bảo toàn khối lượng: \(m_{muối}=m_{KL}+m_{H_2SO_4}-m_{H_2}=68,2\left(g\right)\)

nSO2 = 3.36/22.4 = 0.15 (mol)
nNaOH = 0.5 * 0.1 = 0.05 (mol)
nNaOH / nSO2 = 0.05 / 0.15 = 0.3
Chỉ tạo ra muối axit
NaOH + SO2 => NaHSO3
0.05.........0.05.........0.05
mNaHSO3 = 0.05 * 104 = 5.2 (g)

\(n_{SO_2} = \dfrac{4,48}{22,4} = 0,2(mol) ; n_{NaOH} = 0,25(mol)\\ 1<\dfrac{n_{NaOH}}{n_{SO_2}} = \dfrac{0,25}{0,2} = 1,25 <2 \to Muối\ tạo\ thành : NaHSO_3(a\ mol) ; Na_2SO_3(b\ mol)\\ n_{SO_2} = a + b = 0,2(mol)\\ n_{NaOH} = a + 2b = 0,25(mol)\\ \Rightarrow a = 0,15 ; b = 0,05\\ m_{NaHSO_3} = 0,15.104 = 15,6(gam) \\ m_{Na_2SO_3} = 0,05.126 = 6,3(gam)\)

Đáp án C
Số mol các chất là:
n SO 2 = 0 , 6 mol n NaOH = 0 , 6 . 2 , 5 = 1 , 5 mol n NaOH n SO 2 = 1 , 5 0 , 6 = 2 , 5 > 2
=> Tạo Na2SO3 ⇒ SO 2 : hết NaOH : dư
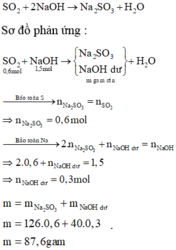

Câu 1:
Đặt \(n_{Fe}=x\left(mol\right);n_{Al}=y\left(mol\right)\)
\(n_{HCl}=0,4\left(mol\right)\)
\(Fe^o\rightarrow Fe^{+2}+2e\)
x_____________2x_(mol)
\(Al^o\rightarrow Al^{+3}+3e\)
y____________3y_(mol)
\(2H^-\rightarrow H_2^o+2e\)
0,8_____0,4____0,8_(mol)
\(BTe:2x+3y=0,8\)
Theo đề ta có hệ: \(\left\{{}\begin{matrix}56x+27y=11\\2x+3y=0,8\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}x=0,1\\y=0,2\end{matrix}\right.\)
\(\left\{{}\begin{matrix}\%m_{Fe}=\frac{0,1.56}{11}.100\%=51\left(\%\right)\\\%m_{Al}=100-51=49\left(\%\right)\end{matrix}\right.\)
\(BTNT:\Rightarrow\left\{{}\begin{matrix}n_{FeCl_2}=0,1\left(mol\right)\\n_{AlCl_3}=0,2\left(mol\right)\end{matrix}\right.\)
\(m_{hh}=0,1.127+133,5.0,2=39,4\left(g\right)\)
\(m_{ddHCl}=\frac{36,5.0,8.100}{7,3}=400\left(g\right)\)
Câu 2:
\(n_{H_2}=0,15\left(mol\right)\)
\(PTHH:2Al+6HCl\rightarrow2AlCl_3+3H_2\)
(mol)____0,1____0,3______0,1______0,15__
\(\%m_{Al_2O_3}=\frac{7,8-27.0,1}{7,8}.100\%=65,4\left(\%\right)\)
Câu 3:
\(n_{H_2}=0,1\left(mol\right)\)
\(PTHH:Zn+2HCl\rightarrow ZnCl_2+H_2\)
(mol)_____0,1__________________0,1__
\(\%m_{ZnO}=\frac{10,55-0,1.65}{10,55}.100\%=38,4\left(\%\right)\)
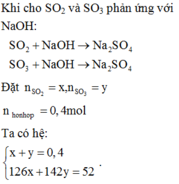
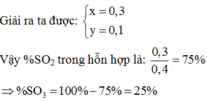



\(n_{SO2}=\frac{0,15}{n_{OH}}=0,5.\left(0,2+0,2\right)\Rightarrow\) Tạo 2 muối
\(n_{SO2/3}=\frac{a}{n_{HSO3}}=b\)
\(\left\{{}\begin{matrix}a+b=0,15\\2a=b=0,2\end{matrix}\right.\Rightarrow\left\{{}\begin{matrix}a=0,05\\b=0,1\end{matrix}\right.\)
mmuối = mNa+ + mK+ + mSO2/3 + mHSO3
\(\Rightarrow m_{muoi}=0,1.23+0,1.39.80+0,1.81=18,3\left(g\right)\)