
K
Khách
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

Các câu hỏi dưới đây có thể giống với câu hỏi trên

5 tháng 5 2023
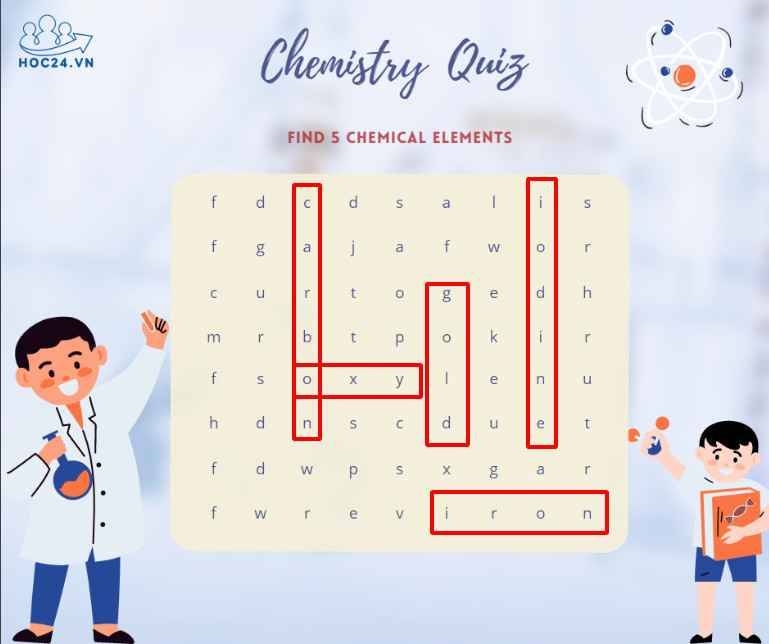
*cái oxy em k chắc á cô:<< em tìm được mấy nguyên tố này th ạ:<.

NK
Nguyễn KIm Ngân
VIP
31 tháng 8
Đề bài cho hỗn hợp X gồm hai khí là methane (CH4) và ethylene (C2H4). Hỗn hợp này có tỉ khối so với hydrogen (H2) bằng 10.
- Bước 1: Tính khối lượng mol trung bình của hỗn hợp X (MX)
Tỉ khối của hỗn hợp X so với hydrogen được tính bằng công thức: dX/H2=MH2MX.
Trong đó, MH2=2 g/mol.
Ta có: 10=2MX⇒MX=10×2=20 g/mol.
- Bước 2: Gọi thành phần phần trăm thể tích của từng khí trong hỗn hợp
Giả sử trong 1 mol hỗn hợp X, số mol của CH4 là x và số mol của C2H4 là y.
Ta có: x+y=1 (1)
Khối lượng mol trung bình của hỗn hợp X được tính bằng công thức: MX=nCH4+nC2H4nCH4⋅MCH4+nC2H4⋅MC2H4.
MCH4=12+4=16 g/mol.
MC2H4=12×2+4=28 g/mol.
Thay vào công thức ta có: 20=x+yx⋅16+y⋅28=116x+28y (2)
Từ (1) và (2) ta có hệ phương trình:
x+y=1
16x+28y=20
Giải hệ phương trình này, ta được: x=32 và y=31.
Điều này có nghĩa là trong hỗn hợp X, số mol của CH4 chiếm 32 và số mol của C2H4 chiếm 31.
- Bước 3: Tính tổng số mol của hỗn hợp X
Thể tích của hỗn hợp X ở điều kiện tiêu chuẩn (đktc) là 3,7185 lít.
Số mol của hỗn hợp X là: nX=24.79V=24.793.7185=0.15 mol.
(Lưu ý: Nếu đề bài cho ở điều kiện tiêu chuẩn cũ (0 độ C, 1 atm) thì V = 22.4, nhưng theo quy ước quốc tế mới, 1 bar và 25 độ C thì V = 24.79. Đề bài không nói rõ nên ta chọn chuẩn mới là 24.79).
- Bước 4: Tính số mol C2H4 trong hỗn hợp X
Từ kết quả ở bước 2, ta biết số mol C2H4 chiếm 31 tổng số mol.
nC2H4=nX×y=0.15×31=0.05 mol.
- Bước 5: Tính số mol Br2 đã phản ứng
Khi hỗn hợp X cho qua dung dịch Br2 dư, chỉ có ethylene (C2H4) phản ứng vì nó có liên kết đôi (C=C). Methane (CH4) là ankan nên không phản ứng với Br2.
Phương trình phản ứng:
C2H4+Br2→C2H4Br2
Theo phương trình, tỉ lệ mol là 1:1, vậy:
nBr2=nC2H4=0.05 mol.
Kết luận
Số mol Br2 đã tham gia phản ứng là 0,05 mol.


HH
6 tháng 11 2017
Câu 3:
N2+O2\(\overset{t^0}{\rightarrow}\)2NO
4NO+3O2+2H2O\(\rightarrow\)4HNO3
NO3- : làm tăng lượng phân đạm cho cây!

JS
7 tháng 9 2017
1. a) Tên gọi của axit:
HNO3: axit nitric
HCl: axit clohidric
H2CO3: axit cacbonic
H2S: axit sunfuhidric
H2SO4: axit sunfuric
H2SO3: axit sunfurơ
Axit mạnh: HCl, HNO3, H2SO4...
Axit yếu: H2S, H2CO3...
NM
0

HR
3

22 tháng 10 2017
Em có thể hỏi bạn Trần Hữu Tuyển. Cô đã chữa cho bạn ấy đề này rồi


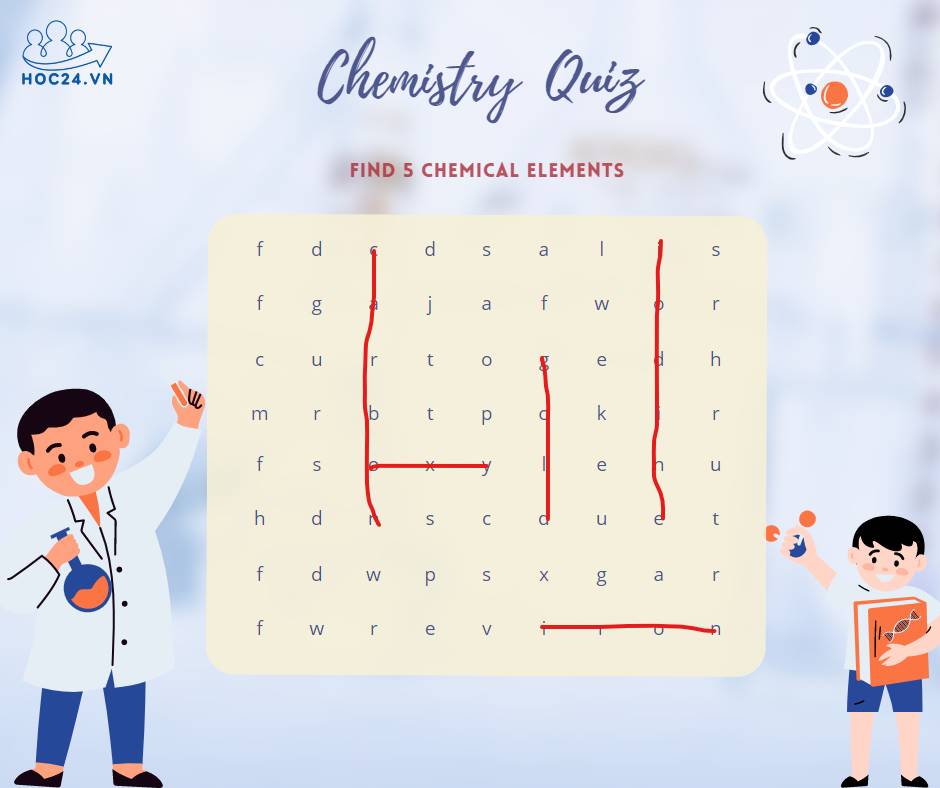














 Giúp mình giải vài câu thực tế Hoá nha..
Giúp mình giải vài câu thực tế Hoá nha..
 Giúp hộ mình nha !!!
Giúp hộ mình nha !!! Cảm ơn bạn !!!
Cảm ơn bạn !!!
 mn giúp e những câu còn lại ạ :)
mn giúp e những câu còn lại ạ :)






$2Na + 2C_2H_5OH \to 2C_2H_5ONa + H_2$
$2Na + 2CH_3COOH \to 2CH_3COONa + H_2$
$CH_3COOH + NaOH \to CH_3COONa + H_2O$
$(C_{17}H_{33}COO)_3C_3H_5 + 3NaOH \to 3C_{17}H_{33}COONa + C_3H_5(OH)_3$
$CaO + 2CH_3COOH \to (CH_3COO)_2Ca + H_2O$
$K_2CO_3 + 2CH_3COOH \to 2CH_3COOK + CO_2 + H_2O$