
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


X: Fe3O4
Y: FeCl2
Z: FeCl3
T: Fe(OH)2
U: Fe(OH)3
A: NaCl (hoặc H2O)
B: H2O (hoặc NaCl)
D: H2 (hoặc Cl2)
E: Cl2 (hoặc H2)
F: NaOH
G: HCl
PTHH:
a) NaCl + H2O -dpmn----> 1/2 H2 + 1/2 Cl2 + NaOH
H2 + Cl2 -to-> 2 HCl
HCl + NaOH -> NaCl + H2O
b) 3 Fe +2 O2 -to->Fe3O4
Fe3O4 + 8 HCl -> FeCl2 +2 FeCl3 + H2O
FeCl2 + 2 NaOH -> Fe(OH)2 + 2 NaCl
FeCl3 +3 NaOH -> Fe(OH)3 + 3NaCl
Chúc em học tốt!

$2Fe + 6H_2SO_4 \to Fe_2(SO_4)_3 + 3SO_2 + 6H_2O(1)$
$Fe_2(SO_4)_3 + Fe\ to 3FeSO_4(2)$
Gọi $n_{Fe_2(SO_4)_3} = a(mol) ; n_{FeSO_4} = b(mol)$
Ta có : $400a + 152b = 62,8(1)$
$n_{SO_2} = 0,15(mol)$
$n_{Fe_2(SO_4)_3(1)} = \dfrac{1}{3}n_{SO_2} = 0,05(mol)$
$n_{Fe_2(SO_4)_3(2)} = \dfrac{1}{3}n_{FeSO_4} = \dfrac{b}{3}$
Suy ra:
$0,05 - \dfrac{b}{3} = a(2)$
Từ (1)(2) suy ra $a = \dfrac{45}{112} ; b = -1,055<0$
=> Sai đề


12.
Na2CO3+H2SO4->Na2SO4+H2O+CO2
............. 0,5 ............. ......... 0,5
CO2+2KOH->K2CO3+H2O
x 2x x
CO2+KOH->KHCO3
y y y
mKOH=98.40/100=39,2g
nKOH=39,2/56=0,7mol
Có:
2x+y=0,7
138x+100y=57,6
=>x=0,2mol; y=0,3mol
mK2CO3=138.0,2=27,6g
mKHCO3=57,6-27,6=30g
b.
nCO2=x+y=0,2+0,3=0,5mol
CMddH2SO4=0,5/0,2=2,5M
8. Hoàn thành sơ đồ chuyển hóa sau:
Mg \(\underrightarrow{\left(1\right)}\) MgO \(\underrightarrow{\left(2\right)}\) MgCl2 \(\underrightarrow{\left(3\right)}\) Mg(OH)2 \(\underrightarrow{\left(4\right)}\) MgO \(\underrightarrow{\left(5\right)}\) MgSO4 \(\underrightarrow{\left(6\right)}\) MgCO3 \(\underrightarrow{\left(7\right)}\) MgO
\(\left(1\right)2Mg+O_2\underrightarrow{t^o}2MgO\)
\(\left(2\right)MgO+2HCl\rightarrow MgCl_2+H_2O\)
\(\left(3\right)MgCl_2+2NaOH\rightarrow Mg\left(OH\right)_2\downarrow+2NaCl\)
\(\left(4\right)Mg\left(OH\right)_2\underrightarrow{t^o}MgO+H_2O\)
\(\left(5\right)MgO+H_2SO_4\rightarrow MgSO_4+H_2O\)
\(\left(6\right)MgSO_4+Na_2CO_3\rightarrow MgCO_3+Na_2SO_4\)
\(\left(7\right)MgCO_3\underrightarrow{t^o}MgO+CO_2\uparrow\)

Đặt CTHH của oxit sắt cần tìm : FexOy
PTHH : FexOy + yH2 = xFe + yH2O
0.2
Theo giả thiết C%H2SO4 còn 98% -3.405%= 94.595%
Hoặc \(\dfrac{98}{100+m_{H2O}}\) =0.94595
giải được mH2O=3.6g
nH2O=0.2 mol
Chất rắn thu được là Fe , nH2 thoát ra=3.36/22.4=0.15 mol
PTHH : Fe + H2SO4 --> FeSO4 + H2
0.15 0.15
Ta có tỉ lệ : nFe:nH2O = x:y = 0,15:0,2 = 3:4
Vậy CTHH của oxit sắt là Fe3O4

https://hoc24.vn/hoi-dap/question/71825.html bạn vào đây tham khảo nè


Kim cương là một dạng thù hình của nguyên tố Cacbon. (Thành phần chính của kim cương là Cacbon). Trong điều kiện áp suất cao và nhiệt độ cao thì các nguyên tử Cacbon kết hợp lại với nhau tạo thành kim cương.
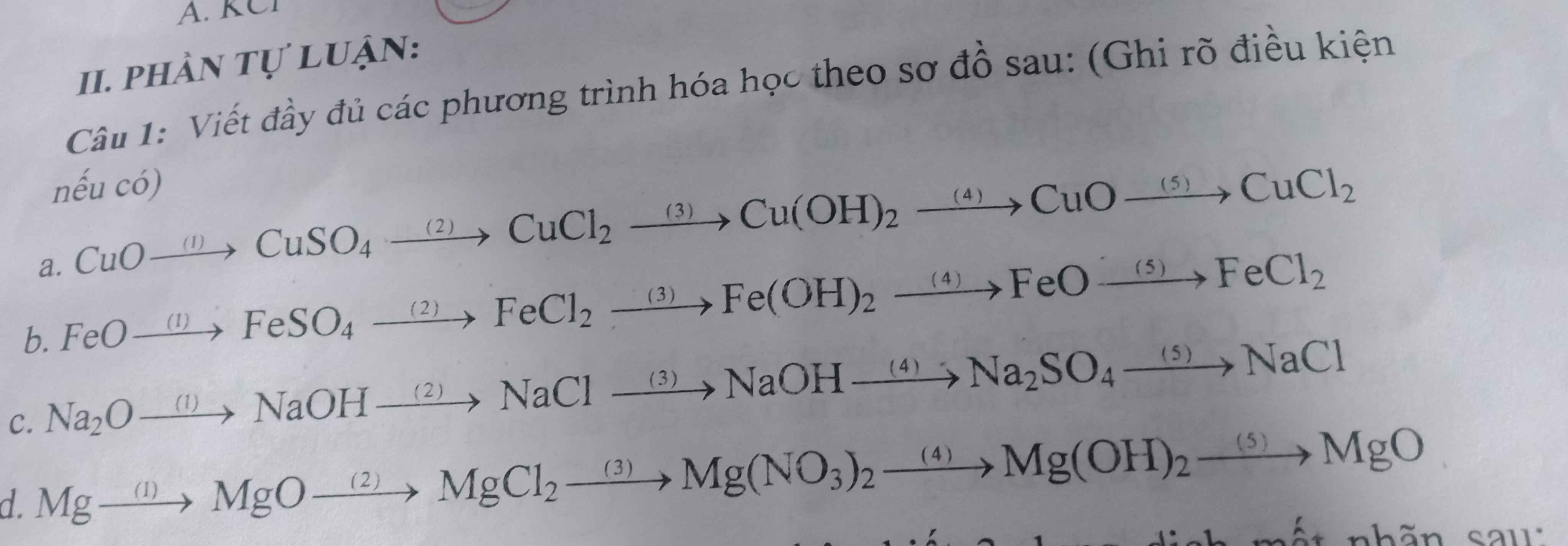

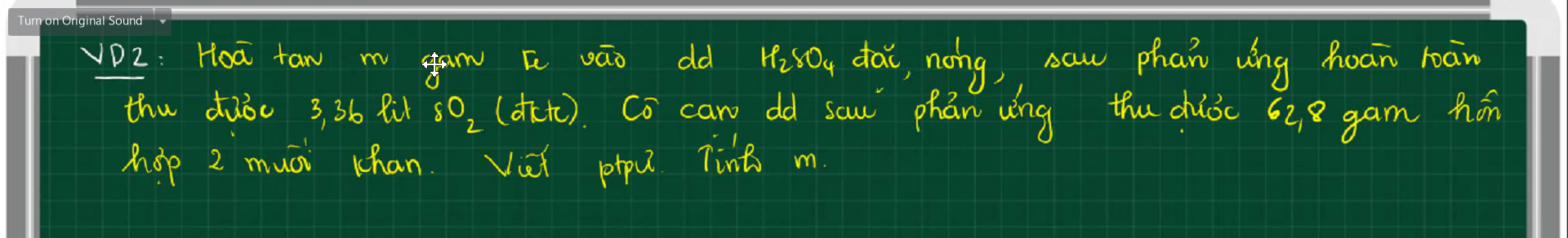

 Giúp hộ mình nha !!!
Giúp hộ mình nha !!!

 Giúp mình nha !!!
Giúp mình nha !!!
a, (1) \(CuO+H_2SO_4\rightarrow CuSO_4+H_2O\)
(2) \(CuSO_4+BaCl_2\rightarrow BaSO_{4\downarrow}+CuCl_2\)
(3) \(CuCl_2+2NaOH\rightarrow Cu\left(OH\right)_{2\downarrow}+2NaCl\)
(4) \(Cu\left(OH\right)_2\underrightarrow{t^o}CuO+H_2O\)
(5) \(CuO+2HCl\rightarrow CuCl_2+H_2O\)
b, (1) \(FeO+H_2SO_4\rightarrow FeSO_4+H_2O\)
(2) \(FeSO_4+BaCl_2\rightarrow FeCl_2+BaSO_{4\downarrow}\)
(3) \(FeCl_2+2NaOH\rightarrow Fe\left(OH\right)_{2\downarrow}+2NaCl\)
(4) \(Fe\left(OH\right)_2\xrightarrow[khongcokk]{t^o}FeO+H_2O\)
(5) \(FeO+2HCl\rightarrow FeCl_2+H_2O\)
c, (1) \(Na_2O+H_2O\rightarrow2NaOH\)
(2) \(NaOH+HCl\rightarrow NaCl+H_2O\)
(3) \(2NaCl+2H_2O\xrightarrow[cmn]{đpdd}2NaOH+Cl_2+H_2\)
(4) \(2NaOH+H_2SO_4\rightarrow Na_2SO_4+2H_2O\)
(5) \(Na_2SO_4+BaCl_2\rightarrow BaSO_{4\downarrow}+2NaCl\)
d, (1) \(Mg+\dfrac{1}{2}O_2\underrightarrow{t^o}MgO\)
(2) \(MgO+2HCl\rightarrow MgCl_2+H_2O\)
(3) \(MgCl_2+2AgNO_3\rightarrow Mg\left(NO_3\right)_2+2AgCl_{\downarrow}\)
(4) \(Mg\left(NO_3\right)_2+2NaOH\rightarrow2NaNO_3+Mg\left(OH\right)_{2\downarrow}\)
(5) \(Mg\left(OH\right)_2\underrightarrow{t^o}MgO+H_2O\)