
Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.


1 đốt
2 cô cạn
3 2,3
4 hạt proton
5 đơn vị cacbon ( đvc )
6 proton electron
7 electron
8 4 . 48335 x 10-23
9 số hạt proton bằng số hạt electron
10 vì khối lượng của electron ko đáng kể
11 proton , nơtron , electron
12 có cùng số proton trog hạt nhân (các nguyên tử cùng loại )
13 sắt , chì , kẽm , thủy ngân
14 Oxi , nitơ , cacbon , clo
15 2 đơn chất 4 hợp chất
16 Fe , O2 , Cl2 , P , Na
17 Na2O , HNO3 , CO2 , CaO , BaCl2
18 342 đvc
19 2O2
20 HNO3
21 P2O5
22 2 nguyên tử Al , 3 nguyên tử S , 4 nguyên tử O
23 CaO , Al2O3 , K2OO
24 Ba3 (PO4)2
25 CO3
26 XY
27 X3Y2
bn nhé

Câu 6:
nAl=3,24/27=0,12(mol); nO2= 4,48/22,4=0,2(mol)
PTHH: 4 Al + 3 O2 -to-> 2 Al2O3
Ta có: 0,12/4 < 0,2/3
=> O2 dư, Al hết, tính theo nAl
=> nAl2O3(LT)= nAl/2= 0,12/2=0,06(mol)
nAl2O3(TT)=4,59/102=0,045(mol)
=> H= (0,045/0,06).100= 75%
Câu 7:
nMg=6/24=0,25(mol); nS= 8,8/32=0,275(mol)
PTHH: Mg + S -to-> MgS
Ta có: 0,25/1 < 0,275/1
=> Mg hết, S dư, tính theo nMg
=> nMgS(LT)=nMg= 0,25(mol)
nMgS(TT)= 10,08/56= 0,18(mol)
=>H= (0,18/0,25).100=72%

(1) K + O2 \(-^{t0}->K2O\)
(2) \(K2O+H2SO4->K2SO4+H2O\)
(4) \(K2SO4+Ba\left(OH\right)2->2KOH+B\text{aS}O4\downarrow\)
\(\left(5\right)KOH+HCl->KCl+H2O\)
\(\left(6\right)2KCl+2H2O\xrightarrow[\text{đ}i\text{ện}-ph\text{â}n]{c\text{ó}-m\text{àng}-ng\text{ă}n}2KOH+Cl2\uparrow+H2\uparrow\)
\(\left(7\right)KOH+Al\left(OH\right)3->KAlO2+2H2O\)
Cái thứ 8 chưa làm bao h :- ?

Bản tường trình
|
Tên thí nghiệm |
Mục đích thí nghiệm |
Hiện tượng | Kết luận |
| Tách riêng chất từ hỗn hợp muối ăn và cát | Biết cách tách riêng chất từ hỗn hợp hai chất |
+) Muối tan trong nước, cát không tan +) Cát được tách riêng trên giấy lọc +)Khi đun, lượng nước bay hơi từ từ, ta được muối tinh khiết hơn muối ban đầu |
-Tách riêng được muối và cát. -Thu được muối tinh khiết |

Bài này dễ em tự làm được mà, nhớ lại các tính chất hóa học của kim loại và oxit là giải quyết được.
P/s: Chữ đẹp v~ =]]

Phần tự luận
Câu 3
a, * Phần tính toán :
Theo đề bài ta có
Số mol của MgSO4 có hòa tan trong 100 ml dung dịch MgSO4 0,4M là
nMgSO4= CM.V=\(\dfrac{100.0,4}{1000}=0,04mol\)
Thể tích dung dịch MgSO4 2M là
VddMgSO4=\(\dfrac{n}{CM}=\dfrac{0,04}{2}=0,02l=20ml\)
*Cách pha chế :
Đong lấy 20ml dung dịch MgSO4 2M cho vào lọ tam giác chó chia vạch và có dung tích là 200ml .Thêm từ từ nước cất vào lọ đến vạch 100ml , lắc đều ta được 100ml dung dịch MgSO4 0,4 M
b, *Cách tính toán :
Theo đề bài ta có
Khối lượng chất tan MgSO4 có trong 250g dd MgSO4 0,1% là
mMgSO4=\(\dfrac{C\%.m\text{dd}}{100\%}\)=\(\dfrac{0,1\%.250}{100\%}=0,25g\)
Khối lượng dung môi ( nước ) cần dùng để pha chế là
mdm=mdd-mct=250-0,25=249,75 g
*Cách pha chế : Cân lấy 0,25g MgSO4 cho vào cốc thủy tinh có chia vạch và có dung tích là 300 ml . Cân lấy 249,75g nước cất hoặc đong lấy 249,75 ml nước cất đổ vào cốc dùng đũa thủy tinh khuấy đều cho tan hết ta được 250g dd MgSO4 0,1%
A TRẮC NGHIỆM
1a 2d 3b 4b
5. 1-a 2-d 3-b 4-c
B TỰ LUẬN
câu 1:
HCl, Ca(OH)2,BẠC NITRAT, SẮT 3 OXIT
cÂU 2;
A)PTPỨ
Zn + 2HCl ------> ZnCl2 + H2
1 2 1 1
b)mHCl=C%*mdd/100=7.3*50/100=3.65g
nHCl=3.65/65=0.05mol
nZn=0.05*1/2=0.025 mol
mZn=0.025*65=3.25g
c)nH2=0.025MOL
VH2=0.025*22.4=0.56 lít
D)nZNCl2=0.025mol
mzncl2=0.025*136=3.4g
sai đó đug chép

haizzzzzzzz
it depends on money we earn .................


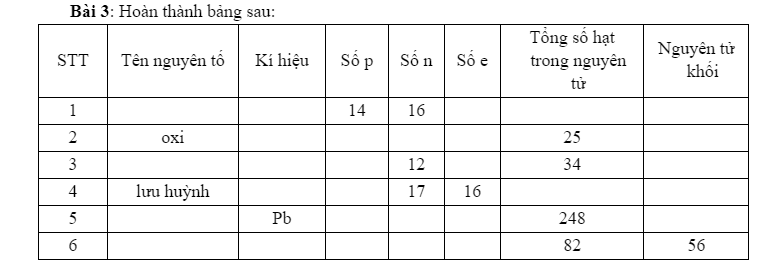
























1. Silic - Si - 14 - 16 - 14 - 44 - 28
2. Oxy - O - 8 - 9 - 8 - 25 - 16
3. Natri - Na - 11 - 12 - 11 - 23
4. Lưu huỳnh - S - 16 - 17 - 16 - 49 - 32
5. Chì - Pb - 82 - 84 - 82 - 248 - 207
6. Sắt - Fe - 26 - 30 - 26 - 82 - 56