Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{Mg}=\dfrac{2,4}{24}=0,1\left(mol\right)\\ a,2Mg+O_2\rightarrow\left(t^o\right)2MgO\\ n_{O_2}=\dfrac{0,1}{2}=0,05\left(mol\right)\\ V_{O_2\left(đktc\right)}=0,05.22,4=1,12\left(l\right)\\ b,2KClO_3\rightarrow\left(t^o\right)2KCl+3O_2\\ n_{KClO_3}=\dfrac{0,05.2}{3}=\dfrac{1}{30}\left(mol\right)\\ \Rightarrow m_{KClO_3}=\dfrac{122,5}{30}=\dfrac{49}{12}\left(g\right)\)

Theo gt ta có: $n_{Mg}=0,15(mol)$
a, $2Mg+O_2\rightarrow 2MgO$
Ta có: $n_{O_2}=0,5.n_{Mg}=0,075(mol)\Rightarrow V_{O_2}=1,68(l)$
b, $2KClO_3\rightarrow 2KCl+3O_2$ (đk: nhiệt độ, MnO2)
Ta có: $n_{KClO_3}=\frac{2}{3}.n_{O_2}=0,05(mol)\Rightarrow m_{KClO_3}=6,125(g)$
\(n_{Mg}=\dfrac{3.6}{24}=0.15\left(mol\right)\)
\(2Mg+O_2\underrightarrow{t^0}2MgO\)
\(0.15......0.075......0.15\)
\(V_{O_2}=0.075\cdot22.4=1.68\left(l\right)\)
\(2KClO_3\underrightarrow{t^0}2KCl+3O_2\)
\(0.05.......................0.075\)
\(m_{KClO_3}=0.05\cdot122.5=6.125\left(g\right)\)

a)
\(n_{Mg}=\dfrac{12}{24}=0,5\left(mol\right)\)
PTHH: 2Mg + O2 --to--> 2MgO
______0,5-->0,25---->0,5
=> VO2 = 0,25.22,4 = 5,6 (l)
=> mMgO = 0,5.40 = 20 (g)
b)
\(n_{O_2}=0,25=>n_{CO_2}=0,25\)
=> mCO2 = 0,25.44 = 11 (g)

a, \(2Mg+O_2\underrightarrow{^{t^o}}2MgO\)
\(n_{MgO}=\dfrac{2}{40}=0,05\left(mol\right)\)
\(n_{O_2}=\dfrac{1}{2}n_{MgO}=0,025\left(mol\right)\Rightarrow V_{O_2}=0,025.22,4=0,56\left(l\right)\)
b, Có lẽ đề cho oxi tác dụng với hidro chứ không phải oxit bạn nhỉ?
\(n_{H_2}=\dfrac{3,36}{22,4}=0,15\left(mol\right)\)
PT: \(2H_2+O_2\underrightarrow{^{t^o}}2H_2O\)
Xét tỉ lệ: \(\dfrac{0,15}{2}>\dfrac{0,025}{1}\), ta được H2 dư.
THeo PT: \(n_{H_2O}=2n_{O_2}=0,05\left(mol\right)\Rightarrow m_{H_2O}=0,05.18=0,9\left(g\right)\)

nAl=16,2/27= 0,6(mol)
a) PTHH: 4 Al +3 O2 -to-> 2 Al2O3
nO2= 3/4 . nAl=3/4 . 0,6= 0,45(mol)
=> V(O2,đktc)=0,45 x 22,4=10,08(l)
b) nAl2O3= nAl/2=0,6/2=0,3(mol)
=>mAl2O3=102. 0,3= 30,6(g)
c) 2KMnO4 -to-> K2MnO4 + MnO2 + O2
nKMnO4= 2.nO2=2. 0,45=0,9(mol)
=>mKMnO4= 158 x 0,9= 142,2(g)

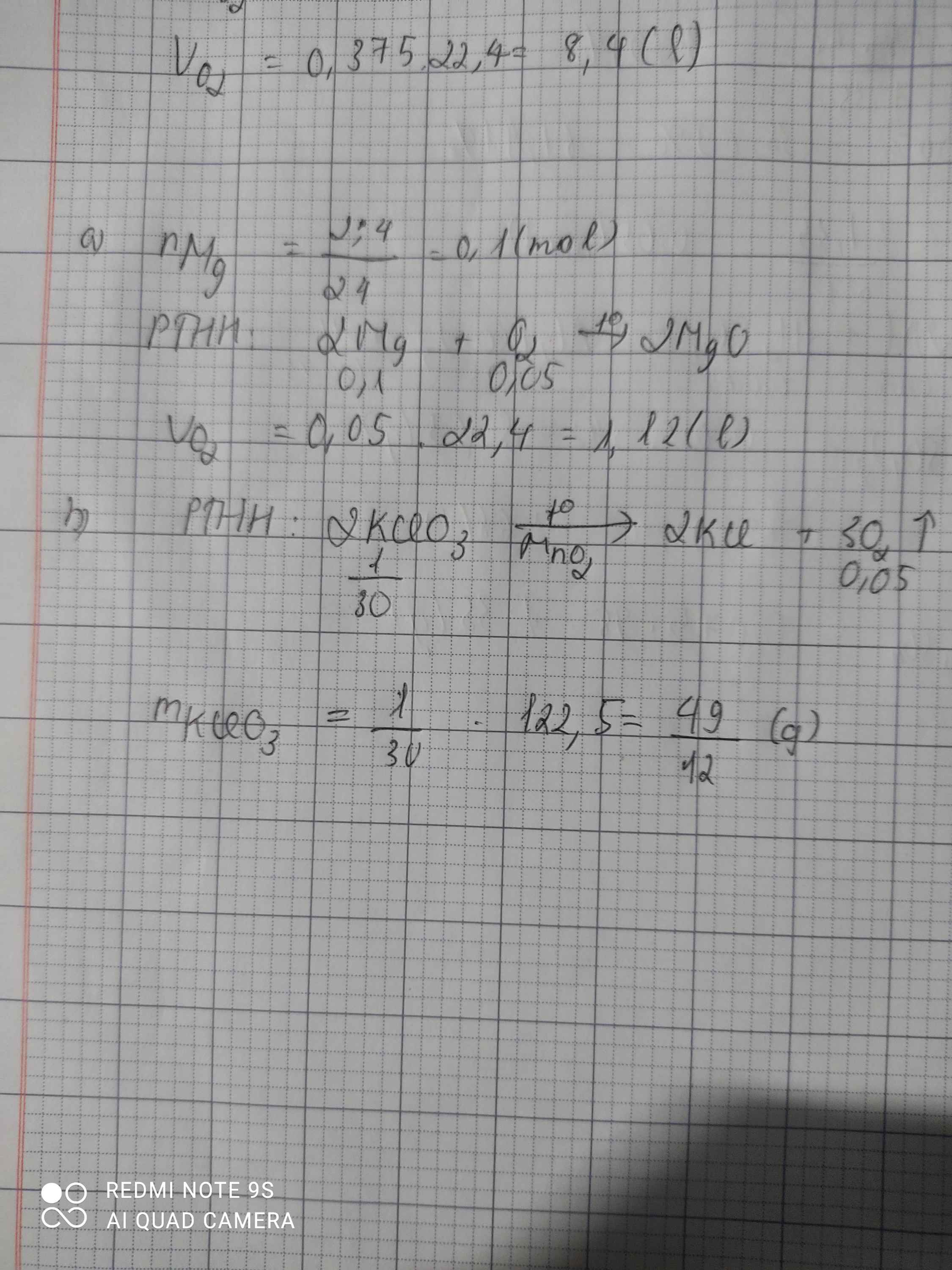


mMg = 3.6/24 = 0.15 (mol)
2Mg + O2 -to-> 2MgO
0.15__0.075____0.15
mMgO= 0.15*40 = 6 (g)
VO2 = 0.075*22.4 = 1.68 (l)
2KClO3 -to-> 2KCl + 3O2
0.05_______________0.075
mKClO3 = 0.05*122.5 = 6.125 (g)
PTHH: \(2Mg+O_2\underrightarrow{t^o}2MgO\)
a+b) Ta có: \(n_{Mg}=\dfrac{3,6}{24}=0,15\left(mol\right)\)
\(\Rightarrow\left\{{}\begin{matrix}n_{O_2}=0,075\left(mol\right)\\n_{MgO}=0,15\left(mol\right)\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}V_{O_2}=0,075\cdot22,4=1,68\left(l\right)\\m_{MgO}=0,15\cdot40=6\left(g\right)\end{matrix}\right.\)
c) PTHH: \(2KClO_3\xrightarrow[MnO_2]{t^o}2KCl+3O_2\uparrow\)
Theo PTHH: \(n_{KClO_3}=0,05\left(mol\right)\)
\(\Rightarrow m_{KClO_3}=0,05\cdot122,5=6,125\left(g\right)\)