Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a)
\(n_{O_2}=\dfrac{3,36}{22,4}=0,15\left(mol\right)\)
\(n_{H_2O}=\dfrac{1,8}{18}=0,1\left(mol\right)\)
PTHH: 2H2 + O2 --to--> 2H2O
0,1<-0,05<-------0,1
2CO + O2 --to--> 2CO2
0,2<--0,1-------->0,2
=> \(\left\{{}\begin{matrix}V_{H_2}=0,1.22,4=2,24\left(l\right)\\V_{CO}=0,2.22,4=4,48\left(l\right)\end{matrix}\right.\)
b) \(m_{CO_2}=0,2.44=8,8\left(g\right)\)
\(n_{O_2}=\dfrac{3,36}{22,4}=0,15mol\)
\(n_{H_2O}=\dfrac{1,8}{18}=0,1mol\)
\(2CO+O_2\rightarrow2CO_2\)
a 0,5a a
\(2H_2+O_2\rightarrow2H_2O\)
0,1 0,05 \(\leftarrow\) 0,1
\(\Sigma n_{O_2}=0,5a+0,05=0,15\)
\(\Rightarrow a=n_{O_2\left(CO\right)}=0,2mol\)
\(V_{CO}=2\cdot0,2\cdot22,4=8,96l\)
\(V_{H_2}=0,1\cdot22,4=2,24l\)
\(m_{CO_2}=0,2\cdot44=8,8g\)

PTHH: CH4 + 2O2 → CO2 ↑ + 2H2O
2C4H10 + 13O2 → 8CO2 ↑ + 10H2O
( Gọi a là số mol của CH4 và 2b là số mol của C4H10 => Số mol của CO2 ở pt (1) là: a và số mol CO2 ở pt (2) là: 8b )
Theo đề bài ra ta có hệ phương trình sau:
16a + 58. 2b = 3,7
44a + 44. 8b = 11
=> a = 0,05 ; b = 0,025
Khối lượng của khí metan trong hỗn hợp ban đầu là:
16 . 0,05 = 0,8 (gam)
Khối lượng của khí butan trong hỗn hợp ban đầu là:
58 . 2. 0,025 = 2,9 (gam)

Sai đề rồi hay sao á bạn, sửa 49,6l thành 89,6l nhé!
a. PTHH: \(2H_2+O_2\rightarrow2H_2O\\ xmol:\dfrac{x}{2}mol\rightarrow xmol\)
\(2CO+O_2\rightarrow2CO_2\\ ymol:\dfrac{y}{2}mol\rightarrow ymol\)
b. Gọi x là số mol của \(H_2\) , y là số mol của \(CO\)
\(m_{hh}=m_{H_2}+m_{CO}\Leftrightarrow2x+28y=68\left(g\right)\left(1\right)\)
\(n_{O_2}=\dfrac{89,6}{22,4}=4\left(mol\right)\Leftrightarrow\dfrac{x}{2}+\dfrac{y}{2}=4\left(mol\right)\)
\(\Leftrightarrow x+y=8\left(2\right)\)
Giải (1) và (2) ta được: \(\left\{{}\begin{matrix}x=6\\y=2\end{matrix}\right.\)
\(\Leftrightarrow\left\{{}\begin{matrix}V_{H_2}=22,4.6=134,4\left(l\right)\\V_{CO}=22,4.2=44,8\left(l\right)\end{matrix}\right.\)
\(\Leftrightarrow\left\{{}\begin{matrix}\%V_{H_2}=\dfrac{134,4}{134,4+44,8}.100\%=75\%\\V_{CO}=25\%\end{matrix}\right.\)

Gọi: \(\left\{{}\begin{matrix}n_{CO}=x\left(mol\right)\\n_{H_2}=y\left(mol\right)\end{matrix}\right.\)
⇒ 28x + 2y = 11,8 (1)
PT: \(2CO+O_2\underrightarrow{t^o}2CO_2\)
\(2H_2+O_2\underrightarrow{t^o}2H_2O\)
Ta có: \(n_{O_2}=\dfrac{7,84}{22,4}=0,35\left(mol\right)\)
Theo PT: \(n_{O_2}=\dfrac{1}{2}n_{CO}+\dfrac{1}{2}n_{H_2}=\dfrac{1}{2}x+\dfrac{1}{2}y\left(mol\right)\)
⇒ x + y = 0,7 (2)
Từ (1) và (2) ⇒ x = 0,4 (mol), y = 0,3 (mol)
a, \(\left\{{}\begin{matrix}\%m_{CO}=\dfrac{0,4.28}{11,8}.100\%\approx94,9\%\\\%m_{H_2}\approx5,1\%\end{matrix}\right.\)
b, Ở cùng điều kiện nhiệt độ và áp suất, % số mol cũng là % thể tích.
\(\Rightarrow\left\{{}\begin{matrix}\%V_{CO}=\dfrac{0,4}{0,7}.100\%\approx57,14\%\\\%V_{H_2}\approx42,86\%\end{matrix}\right.\)
Bạn tham khảo nhé!
PTHH:
\(2CO+O_2\overset{t^o}{--->}2CO_2\left(1\right)\)
\(2H_2+O_2\overset{t^o}{--->}2H_2O\left(2\right)\)
Ta có: \(n_{O_2}=\dfrac{7,84}{22,4}=0,35\left(mol\right)\)
Gọi x, y lần lượt là số mol của CO và H2
a. Theo PT(1): \(n_{O_2}=\dfrac{1}{2}.n_{CO}=\dfrac{1}{2}x\left(mol\right)\)
Theo PT(2): \(n_{O_2}=\dfrac{1}{2}.n_{H_2}=\dfrac{1}{2}y\left(mol\right)\)
\(\Rightarrow\dfrac{1}{2}x+\dfrac{1}{2}y=0,35\) (*)
Theo đề, ta có: \(28x+2y=11,8\) (**)
Từ (*) và (**), ta có HPT:
\(\left\{{}\begin{matrix}\dfrac{1}{2}x+\dfrac{1}{2}y=0,35\\28x+2y=11,8\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}x=0,4\\y=0,3\end{matrix}\right.\)
\(\Rightarrow m_{H_2}=2.0,3=0,6\left(g\right)\)
\(\Rightarrow\%_{m_{H_2}}=\dfrac{0,6}{11,8}.100\%=5,08\%\)
\(\%_{m_{CO}}=100\%-5,08\%=94,92\%\)
b. \(\%_{V_{CO}}=\dfrac{0,4}{0,4+0,3}.100\%=57,1\%\)
\(\%_{V_{H_2}}=100\%-57,1\%=42,9\%\)

Gọi \(\left\{{}\begin{matrix}n_{H_2}=a\left(mol\right)\\n_{CO}=b\left(mol\right)\end{matrix}\right.\)⇒ 2a + 28b = 6,8(1)
\(2H_2 + O_2 \xrightarrow{t^o} 2H_2O\\ 2CO + O_2 \xrightarrow{t^o} 2CO_2\)
Theo PTHH :
\(n_{O_2} = 0,5a + 0,5b = \dfrac{8,96}{22,4} = 0,4(2)\)
Từ (1)(2) suy ra: a = 0,6 ; b = 0,2
Vậy :
\(\%m_{H_2} = \dfrac{0,6.2}{6,8}.100\% = 17,65\%\\ \%m_{CO} = 100\% - 17,65\% = 82,35\%\)
Cho em hỏi tại sao no2=0.5a+0.5b=0.4
tại sao viết 0.5 mà ko là 1 ạ

a) PTHH: \(2CO+O_2\underrightarrow{t^o}2CO_2\) (1)
\(4H_2+O_2\underrightarrow{t^o}2H_2O\) (2)
b) Ta có: \(\left\{{}\begin{matrix}\Sigma n_{O_2}=\dfrac{9,6}{32}=0,3\left(mol\right)\\n_{CO_2}=\dfrac{8,8}{44}=0,2\left(mol\right)\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}n_{O_2\left(1\right)}=0,1mol\\n_{O_2\left(2\right)}=0,2mol\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}m_{CO}=0,1\cdot28=2,8\left(g\right)\\m_{H_2}=0,2\cdot2=0,4\left(g\right)\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}\%m_{CO}=\dfrac{2,8}{2,8+0,4}\cdot100\%=87,5\%\\\%m_{H_2}=12,5\%\end{matrix}\right.\)
c) PTHH: \(2KMnO_4\underrightarrow{t^o}K_2MnO_4+MnO_2+O_2\uparrow\)
Theo PTHH: \(n_{KMnO_4}=2n_{O_2}=0,6mol\)
\(\Rightarrow m_{KMnO_4}=0,6\cdot158=94,8\left(g\right)\)
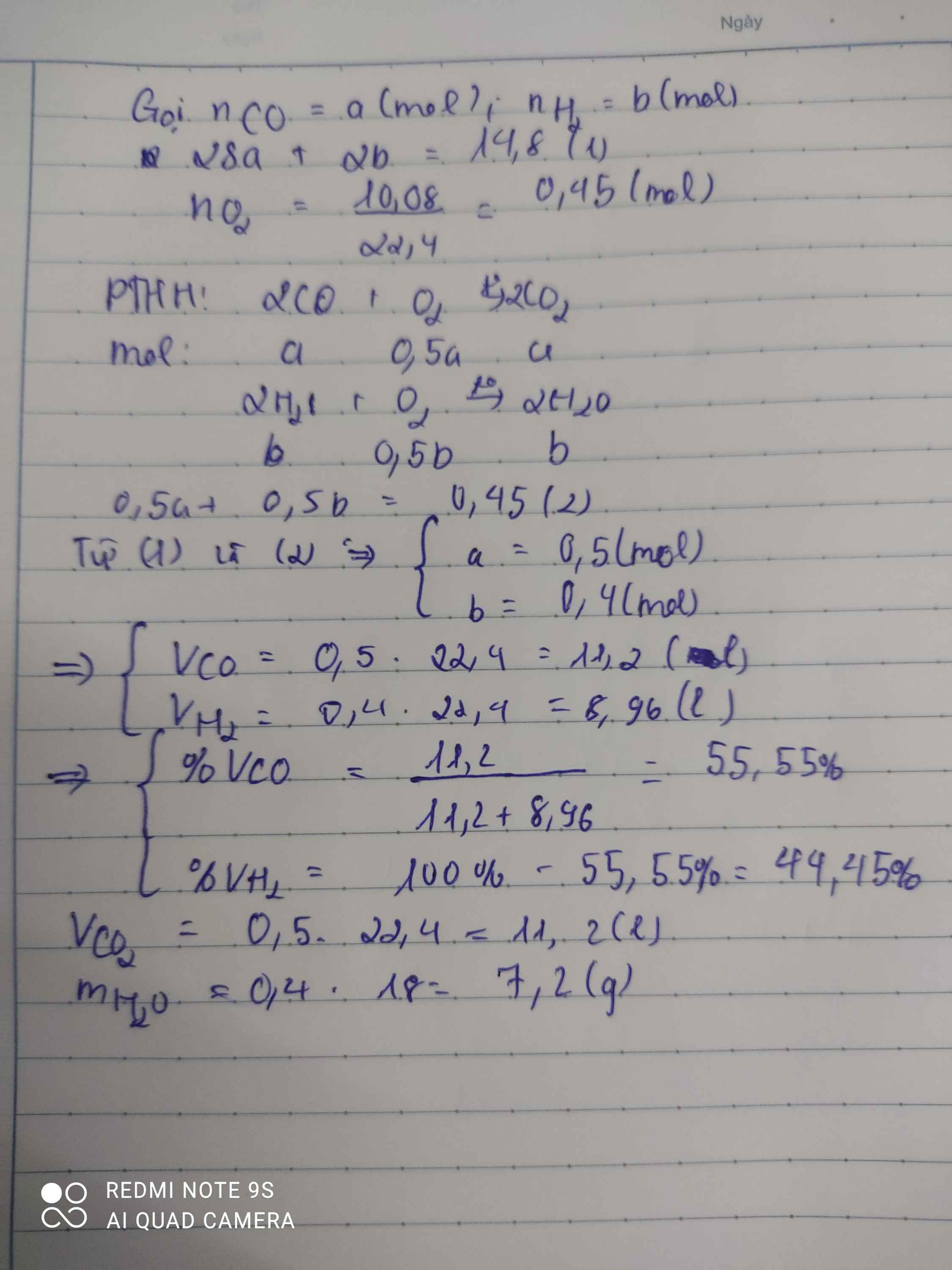
a/ \(2CO\left(0,2\right)+O_2\left(0,1\right)\rightarrow2CO_2\left(0,2\right)\)
\(2H_2\left(0,1\right)+O_2\left(0,05\right)\rightarrow2H_2O\left(0,1\right)\)
\(n_{H_2O}=\frac{1,8}{18}=0,1\)
\(n_{O_2}=\frac{3,36}{22,4}=0,15\)
Số mol O2 phản ứng ở phản ứng đầu là: \(0,15-0,05=0,1\)
\(\Rightarrow m_{CO_2}=0,2.44=8,8\)
b/ \(m_{CO}=0,2.28=5,6\)
\(m_{H_2}=0,1.2=0,2\)
c/ \(\%CO=\frac{0,2}{0,3}.100\%=66,67\%\)
\(\Rightarrow\%H_2=100\%-66,67\%=33,33\%\)